[English] 日本語
 Yorodumi
Yorodumi- PDB-8txo: E. coli DNA-directed RNA polymerase transcription elongation comp... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8txo | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | E. coli DNA-directed RNA polymerase transcription elongation complex bound to the unnatural dZ-PTP base pair in the active site | |||||||||||||||||||||||||||||||||
 Components Components |
| |||||||||||||||||||||||||||||||||
 Keywords Keywords |  TRANSCRIPTION / TRANSCRIPTION /  complex / unnatural base pairs / complex / unnatural base pairs /  synthetic biology synthetic biology | |||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationsubmerged biofilm formation / cellular response to cell envelope stress / cytosolic DNA-directed RNA polymerase complex / regulation of DNA-templated transcription initiation / bacterial-type flagellum assembly / bacterial-type flagellum-dependent cell motility / nitrate assimilation / transcription elongation factor complex / regulation of DNA-templated transcription elongation / transcription antitermination ...submerged biofilm formation / cellular response to cell envelope stress / cytosolic DNA-directed RNA polymerase complex / regulation of DNA-templated transcription initiation / bacterial-type flagellum assembly / bacterial-type flagellum-dependent cell motility / nitrate assimilation / transcription elongation factor complex / regulation of DNA-templated transcription elongation / transcription antitermination /  cell motility / DNA-templated transcription initiation / cell motility / DNA-templated transcription initiation /  ribonucleoside binding / DNA-directed 5'-3' RNA polymerase activity / ribonucleoside binding / DNA-directed 5'-3' RNA polymerase activity /  DNA-directed RNA polymerase / response to heat / intracellular iron ion homeostasis / DNA-directed RNA polymerase / response to heat / intracellular iron ion homeostasis /  protein dimerization activity / response to antibiotic / magnesium ion binding / protein dimerization activity / response to antibiotic / magnesium ion binding /  DNA binding / zinc ion binding / DNA binding / zinc ion binding /  membrane / membrane /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||||||||||||||||||||||||||
| Biological species |   Escherichia coli (E. coli) Escherichia coli (E. coli)synthetic construct (others) | |||||||||||||||||||||||||||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.1 Å cryo EM / Resolution: 3.1 Å | |||||||||||||||||||||||||||||||||
 Authors Authors | Aiyer, S. / Shan, Z. / Oh, J. / Wang, D. / Tan, Y.Z. / Lyumkis, D. | |||||||||||||||||||||||||||||||||
| Funding support |  United States, United States,  Singapore, 10items Singapore, 10items
| |||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Overcoming resolution attenuation during tilted cryo-EM data collection. Authors: Sriram Aiyer / Philip R Baldwin / Shi Min Tan / Zelin Shan / Juntaek Oh / Atousa Mehrani / Marianne E Bowman / Gordon Louie / Dario Oliveira Passos / Selena Đorđević-Marquardt / Mario ...Authors: Sriram Aiyer / Philip R Baldwin / Shi Min Tan / Zelin Shan / Juntaek Oh / Atousa Mehrani / Marianne E Bowman / Gordon Louie / Dario Oliveira Passos / Selena Đorđević-Marquardt / Mario Mietzsch / Joshua A Hull / Shuichi Hoshika / Benjamin A Barad / Danielle A Grotjahn / Robert McKenna / Mavis Agbandje-McKenna / Steven A Benner / Joseph A P Noel / Dong Wang / Yong Zi Tan / Dmitry Lyumkis /    Abstract: Structural biology efforts using cryogenic electron microscopy are frequently stifled by specimens adopting "preferred orientations" on grids, leading to anisotropic map resolution and impeding ...Structural biology efforts using cryogenic electron microscopy are frequently stifled by specimens adopting "preferred orientations" on grids, leading to anisotropic map resolution and impeding structure determination. Tilting the specimen stage during data collection is a generalizable solution but has historically led to substantial resolution attenuation. Here, we develop updated data collection and image processing workflows and demonstrate, using multiple specimens, that resolution attenuation is negligible or significantly reduced across tilt angles. Reconstructions with and without the stage tilted as high as 60° are virtually indistinguishable. These strategies allowed the reconstruction to 3 Å resolution of a bacterial RNA polymerase with preferred orientation, containing an unnatural nucleotide for studying novel base pair recognition. Furthermore, we present a quantitative framework that allows cryo-EM practitioners to define an optimal tilt angle during data acquisition. These results reinforce the utility of employing stage tilt for data collection and provide quantitative metrics to obtain isotropic maps. | |||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8txo.cif.gz 8txo.cif.gz | 486.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8txo.ent.gz pdb8txo.ent.gz | 366.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8txo.json.gz 8txo.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/tx/8txo https://data.pdbj.org/pub/pdb/validation_reports/tx/8txo ftp://data.pdbj.org/pub/pdb/validation_reports/tx/8txo ftp://data.pdbj.org/pub/pdb/validation_reports/tx/8txo | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  41695MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-DNA-directed RNA polymerase subunit ... , 3 types, 4 molecules AGIJ
| #1: Protein |  Polymerase / RNAP subunit alpha / RNA polymerase subunit alpha / Transcriptase subunit alpha Polymerase / RNAP subunit alpha / RNA polymerase subunit alpha / Transcriptase subunit alphaMass: 36558.680 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli (E. coli) / Gene: rpoA, pez, phs, sez, b3295, JW3257 / Production host: Escherichia coli (E. coli) / Gene: rpoA, pez, phs, sez, b3295, JW3257 / Production host:   Escherichia coli BL21(DE3) (bacteria) / References: UniProt: P0A7Z4, Escherichia coli BL21(DE3) (bacteria) / References: UniProt: P0A7Z4,  DNA-directed RNA polymerase DNA-directed RNA polymerase#2: Protein | |  Polymerase / RNAP subunit beta / RNA polymerase subunit beta / Transcriptase subunit beta Polymerase / RNAP subunit beta / RNA polymerase subunit beta / Transcriptase subunit betaMass: 150820.875 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli (E. coli) Escherichia coli (E. coli)Gene: rpoB, groN, nitB, rif, ron, stl, stv, tabD, b3987, JW3950 Production host:   Escherichia coli BL21(DE3) (bacteria) / References: UniProt: P0A8V2, Escherichia coli BL21(DE3) (bacteria) / References: UniProt: P0A8V2,  DNA-directed RNA polymerase DNA-directed RNA polymerase#3: Protein | |  Polymerase / RNAP subunit beta' / RNA polymerase subunit beta' / Transcriptase subunit beta' Polymerase / RNAP subunit beta' / RNA polymerase subunit beta' / Transcriptase subunit beta'Mass: 158105.672 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli (E. coli) / Gene: rpoC, tabB, b3988, JW3951 / Production host: Escherichia coli (E. coli) / Gene: rpoC, tabB, b3988, JW3951 / Production host:   Escherichia coli BL21(DE3) (bacteria) / References: UniProt: P0A8T7, Escherichia coli BL21(DE3) (bacteria) / References: UniProt: P0A8T7,  DNA-directed RNA polymerase DNA-directed RNA polymerase |
|---|
-DNA chain , 2 types, 2 molecules NT
| #4: DNA chain | Mass: 5663.694 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
|---|---|
| #6: DNA chain | Mass: 6135.868 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
-RNA chain , 1 types, 1 molecules R
| #5: RNA chain | Mass: 2934.831 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
|---|
-Non-polymers , 3 types, 4 molecules 

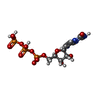


| #7: Chemical | | #8: Chemical | ChemComp-MG / | #9: Chemical | ChemComp-S9F / [[( | |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: E. coli RNAP transcription elongation complex bound the unnatural dZ-PTP base pair in the active site Type: COMPLEX / Entity ID: #1-#6 / Source: MULTIPLE SOURCES |
|---|---|
| Molecular weight | Value: 0.5 MDa / Experimental value: YES |
| Source (natural) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Source (recombinant) | Organism:   Escherichia coli 'BL21-Gold(DE3)pLysS AG' (bacteria) Escherichia coli 'BL21-Gold(DE3)pLysS AG' (bacteria) |
| Buffer solution | pH: 8 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YESDetails: Specimen concentration ranged from 0.1-0.4 mg/ml for data collection |
| Specimen support | Grid material: GOLD / Grid mesh size: 300 divisions/in. / Grid type: UltrAuFoil R1.2/1.3 |
Vitrification | Cryogen name: ETHANE / Humidity: 90 % |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal magnification: 105000 X / Nominal defocus max: 1800 nm / Nominal defocus min: 1000 nm / Cs Bright-field microscopy / Nominal magnification: 105000 X / Nominal defocus max: 1800 nm / Nominal defocus min: 1000 nm / Cs : 2.7 mm / C2 aperture diameter: 50 µm / Alignment procedure: ZEMLIN TABLEAU : 2.7 mm / C2 aperture diameter: 50 µm / Alignment procedure: ZEMLIN TABLEAU |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 0.75 sec. / Electron dose: 35.6 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Num. of grids imaged: 2 / Num. of real images: 527 |
| EM imaging optics | Energyfilter name : GIF Bioquantum / Energyfilter slit width: 20 eV : GIF Bioquantum / Energyfilter slit width: 20 eV |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 72000 | ||||||||||||||||||||||||||||||
| Symmetry | Point symmetry : C1 (asymmetric) : C1 (asymmetric) | ||||||||||||||||||||||||||||||
3D reconstruction | Resolution: 3.1 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 72000 / Symmetry type: POINT | ||||||||||||||||||||||||||||||
| Atomic model building | Protocol: FLEXIBLE FIT / Space: REAL | ||||||||||||||||||||||||||||||
| Atomic model building | Details: used dZ:PTP atomic model / Source name: Other / Type: experimental model | ||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller























 PDBj
PDBj









































