+Search query
-Structure paper
| Title | Structural and dynamic insights into supra-physiological activation and allosteric modulation of a muscarinic acetylcholine receptor. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 14, Issue 1, Page 376, Year 2023 |
| Publish date | Jan 23, 2023 |
 Authors Authors | Jun Xu / Qinggong Wang / Harald Hübner / Yunfei Hu / Xiaogang Niu / Haoqing Wang / Shoji Maeda / Asuka Inoue / Yuyong Tao / Peter Gmeiner / Yang Du / Changwen Jin / Brian K Kobilka /     |
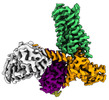
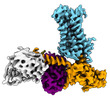
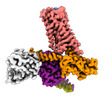
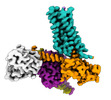
| PubMed Abstract | The M2 muscarinic receptor (M2R) is a prototypical G-protein-coupled receptor (GPCR) that serves as a model system for understanding GPCR regulation by both orthosteric and allosteric ligands. Here, ...The M2 muscarinic receptor (M2R) is a prototypical G-protein-coupled receptor (GPCR) that serves as a model system for understanding GPCR regulation by both orthosteric and allosteric ligands. Here, we investigate the mechanisms governing M2R signaling versatility using cryo-electron microscopy (cryo-EM) and NMR spectroscopy, focusing on the physiological agonist acetylcholine and a supra-physiological agonist iperoxo, as well as a positive allosteric modulator LY2119620. These studies reveal that acetylcholine stabilizes a more heterogeneous M2R-G-protein complex than iperoxo, where two conformers with distinctive G-protein orientations were determined. We find that LY2119620 increases the affinity for both agonists, but differentially modulates agonists efficacy in G-protein and β-arrestin pathways. Structural and spectroscopic analysis suggest that LY211620 stabilizes distinct intracellular conformational ensembles from agonist-bound M2R, which may enhance β-arrestin recruitment while impairing G-protein activation. These results highlight the role of conformational dynamics in the complex signaling behavior of GPCRs, and could facilitate design of better drugs. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:36690613 / PubMed:36690613 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 3.16 - 3.32 Å |
| Structure data | EMDB-25748, PDB-7t8x: EMDB-25749, PDB-7t90: EMDB-25751, PDB-7t94: EMDB-25752, PDB-7t96: |
| Chemicals |  ChemComp-ACH:  ChemComp-HOH:  ChemComp-2CU: |
| Source |
|
 Keywords Keywords |  MEMBRANE PROTEIN / MEMBRANE PROTEIN /  GPCR / GPCR /  signaling complex / muscarinic receptor / signaling complex / muscarinic receptor /  acetylcholine acetylcholine |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers