[English] 日本語
 Yorodumi
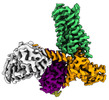
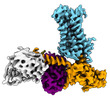
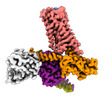
Yorodumi- EMDB-25752: Cryo-EM structure of S2 state ACh-bound M2R-Go signaling complex ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | 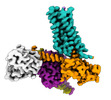 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of S2 state ACh-bound M2R-Go signaling complex with a PAM | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  GPCR / GPCR /  signaling complex / muscarinic receptor / signaling complex / muscarinic receptor /  acetylcholine / acetylcholine /  MEMBRANE PROTEIN MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology information Muscarinic acetylcholine receptors / symmetric synapse / phospholipase C-activating G protein-coupled acetylcholine receptor signaling pathway / G protein-coupled acetylcholine receptor activity / regulation of smooth muscle contraction / cholinergic synapse / adenylate cyclase-inhibiting G protein-coupled acetylcholine receptor signaling pathway / mu-type opioid receptor binding / Muscarinic acetylcholine receptors / symmetric synapse / phospholipase C-activating G protein-coupled acetylcholine receptor signaling pathway / G protein-coupled acetylcholine receptor activity / regulation of smooth muscle contraction / cholinergic synapse / adenylate cyclase-inhibiting G protein-coupled acetylcholine receptor signaling pathway / mu-type opioid receptor binding /  corticotropin-releasing hormone receptor 1 binding / G protein-coupled serotonin receptor activity ... corticotropin-releasing hormone receptor 1 binding / G protein-coupled serotonin receptor activity ... Muscarinic acetylcholine receptors / symmetric synapse / phospholipase C-activating G protein-coupled acetylcholine receptor signaling pathway / G protein-coupled acetylcholine receptor activity / regulation of smooth muscle contraction / cholinergic synapse / adenylate cyclase-inhibiting G protein-coupled acetylcholine receptor signaling pathway / mu-type opioid receptor binding / Muscarinic acetylcholine receptors / symmetric synapse / phospholipase C-activating G protein-coupled acetylcholine receptor signaling pathway / G protein-coupled acetylcholine receptor activity / regulation of smooth muscle contraction / cholinergic synapse / adenylate cyclase-inhibiting G protein-coupled acetylcholine receptor signaling pathway / mu-type opioid receptor binding /  corticotropin-releasing hormone receptor 1 binding / G protein-coupled serotonin receptor activity / arrestin family protein binding / corticotropin-releasing hormone receptor 1 binding / G protein-coupled serotonin receptor activity / arrestin family protein binding /  regulation of heart contraction / dopamine receptor signaling pathway / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / asymmetric synapse / G protein-coupled serotonin receptor binding / axon terminus / presynaptic modulation of chemical synaptic transmission / regulation of heart contraction / dopamine receptor signaling pathway / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / asymmetric synapse / G protein-coupled serotonin receptor binding / axon terminus / presynaptic modulation of chemical synaptic transmission /  muscle contraction / clathrin-coated endocytic vesicle membrane / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / response to virus / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G-protein activation / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / ADP signalling through P2Y purinoceptor 12 / G beta:gamma signalling through BTK / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / photoreceptor disc membrane / Adrenaline,noradrenaline inhibits insulin secretion / Glucagon-type ligand receptors / Vasopressin regulates renal water homeostasis via Aquaporins / G alpha (z) signalling events / cellular response to catecholamine stimulus / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / ADORA2B mediated anti-inflammatory cytokines production / adenylate cyclase-activating dopamine receptor signaling pathway / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / cellular response to prostaglandin E stimulus / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / sensory perception of taste / GPER1 signaling / G-protein beta-subunit binding / muscle contraction / clathrin-coated endocytic vesicle membrane / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / response to virus / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G-protein activation / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / ADP signalling through P2Y purinoceptor 12 / G beta:gamma signalling through BTK / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / photoreceptor disc membrane / Adrenaline,noradrenaline inhibits insulin secretion / Glucagon-type ligand receptors / Vasopressin regulates renal water homeostasis via Aquaporins / G alpha (z) signalling events / cellular response to catecholamine stimulus / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / ADORA2B mediated anti-inflammatory cytokines production / adenylate cyclase-activating dopamine receptor signaling pathway / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / cellular response to prostaglandin E stimulus / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / sensory perception of taste / GPER1 signaling / G-protein beta-subunit binding /  heterotrimeric G-protein complex / Inactivation, recovery and regulation of the phototransduction cascade / heterotrimeric G-protein complex / Inactivation, recovery and regulation of the phototransduction cascade /  extracellular vesicle / G alpha (12/13) signalling events / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / Cargo recognition for clathrin-mediated endocytosis / retina development in camera-type eye / extracellular vesicle / G alpha (12/13) signalling events / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / Cargo recognition for clathrin-mediated endocytosis / retina development in camera-type eye /  GTPase binding / GTPase binding /  presynaptic membrane / presynaptic membrane /  Clathrin-mediated endocytosis / Ca2+ pathway / phospholipase C-activating G protein-coupled receptor signaling pathway / Clathrin-mediated endocytosis / Ca2+ pathway / phospholipase C-activating G protein-coupled receptor signaling pathway /  nervous system development / G alpha (i) signalling events / fibroblast proliferation / chemical synaptic transmission / G alpha (s) signalling events / nervous system development / G alpha (i) signalling events / fibroblast proliferation / chemical synaptic transmission / G alpha (s) signalling events /  postsynaptic membrane / G alpha (q) signalling events / Ras protein signal transduction / cell population proliferation / Extra-nuclear estrogen signaling / G protein-coupled receptor signaling pathway / lysosomal membrane / postsynaptic membrane / G alpha (q) signalling events / Ras protein signal transduction / cell population proliferation / Extra-nuclear estrogen signaling / G protein-coupled receptor signaling pathway / lysosomal membrane /  GTPase activity / GTPase activity /  dendrite / neuronal cell body / glutamatergic synapse / dendrite / neuronal cell body / glutamatergic synapse /  synapse / protein-containing complex binding / GTP binding / synapse / protein-containing complex binding / GTP binding /  signal transduction / extracellular exosome / signal transduction / extracellular exosome /  membrane / membrane /  metal ion binding / metal ion binding /  plasma membrane / plasma membrane /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) / Homo sapiens (human) /   Mus musculus (house mouse) Mus musculus (house mouse) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.22 Å cryo EM / Resolution: 3.22 Å | |||||||||
 Authors Authors | Xu J / Wang Q / Du Y / Kobilka BK | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural and dynamic insights into supra-physiological activation and allosteric modulation of a muscarinic acetylcholine receptor. Authors: Jun Xu / Qinggong Wang / Harald Hübner / Yunfei Hu / Xiaogang Niu / Haoqing Wang / Shoji Maeda / Asuka Inoue / Yuyong Tao / Peter Gmeiner / Yang Du / Changwen Jin / Brian K Kobilka /     Abstract: The M2 muscarinic receptor (M2R) is a prototypical G-protein-coupled receptor (GPCR) that serves as a model system for understanding GPCR regulation by both orthosteric and allosteric ligands. Here, ...The M2 muscarinic receptor (M2R) is a prototypical G-protein-coupled receptor (GPCR) that serves as a model system for understanding GPCR regulation by both orthosteric and allosteric ligands. Here, we investigate the mechanisms governing M2R signaling versatility using cryo-electron microscopy (cryo-EM) and NMR spectroscopy, focusing on the physiological agonist acetylcholine and a supra-physiological agonist iperoxo, as well as a positive allosteric modulator LY2119620. These studies reveal that acetylcholine stabilizes a more heterogeneous M2R-G-protein complex than iperoxo, where two conformers with distinctive G-protein orientations were determined. We find that LY2119620 increases the affinity for both agonists, but differentially modulates agonists efficacy in G-protein and β-arrestin pathways. Structural and spectroscopic analysis suggest that LY211620 stabilizes distinct intracellular conformational ensembles from agonist-bound M2R, which may enhance β-arrestin recruitment while impairing G-protein activation. These results highlight the role of conformational dynamics in the complex signaling behavior of GPCRs, and could facilitate design of better drugs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25752.map.gz emd_25752.map.gz | 111.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25752-v30.xml emd-25752-v30.xml emd-25752.xml emd-25752.xml | 15.8 KB 15.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_25752.png emd_25752.png | 111.7 KB | ||
| Filedesc metadata |  emd-25752.cif.gz emd-25752.cif.gz | 6.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25752 http://ftp.pdbj.org/pub/emdb/structures/EMD-25752 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25752 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25752 | HTTPS FTP |
-Related structure data
| Related structure data |  7t96MC  7t8xC  7t90C  7t94C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25752.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25752.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : ACh-bound M2R-GoA-scFv16 complex
| Entire | Name: ACh-bound M2R-GoA-scFv16 complex |
|---|---|
| Components |
|
-Supramolecule #1: ACh-bound M2R-GoA-scFv16 complex
| Supramolecule | Name: ACh-bound M2R-GoA-scFv16 complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Muscarinic acetylcholine receptor M2
| Macromolecule | Name: Muscarinic acetylcholine receptor M2 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 39.72991 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: DYKDDDDAST DSSDNSLALT SPYKTFEVVF IVLVAGSLSL VTIIGNILVM VSIKVNRHLQ TVNNYFLFSL ACADLIIGVF SMNLYTLYT VIGYWPLGPV VCDLWLALDY VVSNASVMNL LIISFDRYFC VTKPLTYPVK RTTKMAGMMI AAAWVLSFIL W APAILFWQ ...String: DYKDDDDAST DSSDNSLALT SPYKTFEVVF IVLVAGSLSL VTIIGNILVM VSIKVNRHLQ TVNNYFLFSL ACADLIIGVF SMNLYTLYT VIGYWPLGPV VCDLWLALDY VVSNASVMNL LIISFDRYFC VTKPLTYPVK RTTKMAGMMI AAAWVLSFIL W APAILFWQ FIVGVRTVED GECYIQFFSN AAVTFGTAIA AFYLPVIIMT VLYWHISRAS KSRIKKDKKE PVANQDPVSI VA RKIVKMT KQPAKKKPPP SREKKVTRTI LAILLAFIIT WAPYNVMVLI NTFCAPCIPN TVWTIGYWLC YINSTINPAC YAL CNATFK KTFKHLLMCH YKNIGATRPA GLEVLFQ UniProtKB:  Muscarinic acetylcholine receptor M2, Muscarinic acetylcholine receptor M2,  Muscarinic acetylcholine receptor M2 Muscarinic acetylcholine receptor M2 |
-Macromolecule #2: Guanine nucleotide-binding protein G(o) subunit alpha
| Macromolecule | Name: Guanine nucleotide-binding protein G(o) subunit alpha / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.104551 KDa |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MGCTLSAEDK AAVERSKMIE KNLKEDGISA AKDVKLLLLG AGESGKSTIV KQMKIIHEDG FSGEDVKQYK PVVYSNTIQS LAAIVRAMD TLGIEYGDKE RKADAKMVCD VVSRMEDTEP FSAELLSAMM RLWGDSGIQE CFNRSREYQL NDSAKYYLDS L DRIGAADY ...String: MGCTLSAEDK AAVERSKMIE KNLKEDGISA AKDVKLLLLG AGESGKSTIV KQMKIIHEDG FSGEDVKQYK PVVYSNTIQS LAAIVRAMD TLGIEYGDKE RKADAKMVCD VVSRMEDTEP FSAELLSAMM RLWGDSGIQE CFNRSREYQL NDSAKYYLDS L DRIGAADY QPTEQDILRT RVKTTGIVET HFTFKNLHFR LFDVGGQRSE RKKWIHCFED VTAIIFCVAL SGYDQVLHED ET TNRMHES LMLFDSICNN KFFIDTSIIL FLNKKDLFGE KIKKSPLTIC FPEYTGPNTY EDAAAYIQAQ FESKNRSPNK EIY CHMTCA TDTNNIQVVF DAVTDIIIAN NLRGCGLY UniProtKB: Guanine nucleotide-binding protein G(o) subunit alpha |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.728152 KDa |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: GPGSSGSELD QLRQEAEQLK NQIRDARKAC ADATLSQITN NIDPVGRIQM RTRRTLRGHL AKIYAMHWGT DSRLLVSASQ DGKLIIWDS YTTNKVHAIP LRSSWVMTCA YAPSGNYVAC GGLDNICSIY NLKTREGNVR VSRELAGHTG YLSCCRFLDD N QIVTSSGD ...String: GPGSSGSELD QLRQEAEQLK NQIRDARKAC ADATLSQITN NIDPVGRIQM RTRRTLRGHL AKIYAMHWGT DSRLLVSASQ DGKLIIWDS YTTNKVHAIP LRSSWVMTCA YAPSGNYVAC GGLDNICSIY NLKTREGNVR VSRELAGHTG YLSCCRFLDD N QIVTSSGD TTCALWDIET GQQTTTFTGH TGDVMSLSLA PDTRLFVSGA CDASAKLWDV REGMCRQTFT GHESDINAIC FF PNGNAFA TGSDDATCRL FDLRADQELM TYSHDNIICG ITSVSFSKSG RLLLAGYDDF NCNVWDALKA DRAGVLAGHD NRV SCLGVT DDGMAVATGS WDSFLKIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #5: scFV16
| Macromolecule | Name: scFV16 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) |
| Molecular weight | Theoretical: 27.340482 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: DVQLVESGGG LVQPGGSRKL SCSASGFAFS SFGMHWVRQA PEKGLEWVAY ISSGSGTIYY ADTVKGRFTI SRDDPKNTLF LQMTSLRSE DTAMYYCVRS IYYYGSSPFD FWGQGTTLTV SSGGGGSGGG GSGGGGSDIV MTQATSSVPV TPGESVSISC R SSKSLLHS ...String: DVQLVESGGG LVQPGGSRKL SCSASGFAFS SFGMHWVRQA PEKGLEWVAY ISSGSGTIYY ADTVKGRFTI SRDDPKNTLF LQMTSLRSE DTAMYYCVRS IYYYGSSPFD FWGQGTTLTV SSGGGGSGGG GSGGGGSDIV MTQATSSVPV TPGESVSISC R SSKSLLHS NGNTYLYWFL QRPGQSPQLL IYRMSNLASG VPDRFSGSGS GTAFTLTISR LEAEDVGVYY CMQHLEYPLT FG AGTKLEL KGSLEVLFQ |
-Macromolecule #6: ACETYLCHOLINE
| Macromolecule | Name: ACETYLCHOLINE / type: ligand / ID: 6 / Number of copies: 1 / Formula: ACH |
|---|---|
| Molecular weight | Theoretical: 146.207 Da |
| Chemical component information | 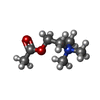 ChemComp-ACH: |
-Macromolecule #7: 3-amino-5-chloro-N-cyclopropyl-4-methyl-6-[2-(4-methylpiperazin-1...
| Macromolecule | Name: 3-amino-5-chloro-N-cyclopropyl-4-methyl-6-[2-(4-methylpiperazin-1-yl)-2-oxoethoxy]thieno[2,3-b]pyridine-2-carboxamide type: ligand / ID: 7 / Number of copies: 1 / Formula: 2CU |
|---|---|
| Molecular weight | Theoretical: 437.944 Da |
| Chemical component information |  ChemComp-2CU: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm Bright-field microscopy / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.22 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 80017 |
 Movie
Movie Controller
Controller