+Search query
-Structure paper
| Title | Structural flexibility at a major conserved antibody target on hepatitis C virus E2 antigen. |
|---|---|
| Journal, issue, pages | Proc Natl Acad Sci U S A, Vol. 113, Issue 45, Page 12768-12773, Year 2016 |
| Publish date | Nov 8, 2016 |
 Authors Authors | Leopold Kong / David E Lee / Rameshwar U Kadam / Tong Liu / Erick Giang / Travis Nieusma / Fernando Garces / Netanel Tzarum / Virgil L Woods / Andrew B Ward / Sheng Li / Ian A Wilson / Mansun Law /  |
| PubMed Abstract | Hepatitis C virus (HCV) is a major cause of liver disease, affecting over 2% of the world's population. The HCV envelope glycoproteins E1 and E2 mediate viral entry, with E2 being the main target of ...Hepatitis C virus (HCV) is a major cause of liver disease, affecting over 2% of the world's population. The HCV envelope glycoproteins E1 and E2 mediate viral entry, with E2 being the main target of neutralizing antibody responses. Structural investigations of E2 have produced templates for vaccine design, including the conserved CD81 receptor-binding site (CD81bs) that is a key target of broadly neutralizing antibodies (bNAbs). Unfortunately, immunization with recombinant E2 and E1E2 rarely elicits sufficient levels of bNAbs for protection. To understand the challenges for eliciting bNAb responses against the CD81bs, we investigated the E2 CD81bs by electron microscopy (EM), hydrogen-deuterium exchange (HDX), molecular dynamics (MD), and calorimetry. By EM, we observed that HCV1, a bNAb recognizing the N-terminal region of the CD81bs, bound a soluble E2 core construct from multiple angles of approach, suggesting components of the CD81bs are flexible. HDX of multiple E2 constructs consistently indicated the entire CD81bs was flexible relative to the rest of the E2 protein, which was further confirmed by MD simulations. However, E2 has a high melting temperature of 84.8 °C, which is more akin to proteins from thermophilic organisms. Thus, recombinant E2 is a highly stable protein overall, but with an exceptionally flexible CD81bs. Such flexibility may promote induction of nonneutralizing antibodies over bNAbs to E2 CD81bs, underscoring the necessity of rigidifying this antigenic region as a target for rational vaccine design. |
 External links External links |  Proc Natl Acad Sci U S A / Proc Natl Acad Sci U S A /  PubMed:27791120 / PubMed:27791120 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 30.0 Å |
| Structure data | 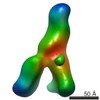 EMDB-8338: 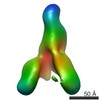 EMDB-8339: 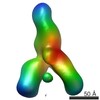 EMDB-8340: |
| Source |
|
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers



 Hepatitis C virus
Hepatitis C virus
 Homo sapiens (human)
Homo sapiens (human)