+Search query
-Structure paper
| Title | Asymmetric conformations and lipid interactions shape the ATP-coupled cycle of a heterodimeric ABC transporter. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 14, Issue 1, Page 7184, Year 2023 |
| Publish date | Nov 8, 2023 |
 Authors Authors | Qingyu Tang / Matt Sinclair / Hale S Hasdemir / Richard A Stein / Erkan Karakas / Emad Tajkhorshid / Hassane S Mchaourab /  |
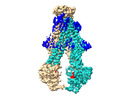
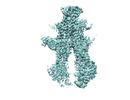
| PubMed Abstract | Here we used cryo-electron microscopy (cryo-EM), double electron-electron resonance spectroscopy (DEER), and molecular dynamics (MD) simulations, to capture and characterize ATP- and substrate-bound ...Here we used cryo-electron microscopy (cryo-EM), double electron-electron resonance spectroscopy (DEER), and molecular dynamics (MD) simulations, to capture and characterize ATP- and substrate-bound inward-facing (IF) and occluded (OC) conformational states of the heterodimeric ATP binding cassette (ABC) multidrug exporter BmrCD in lipid nanodiscs. Supported by DEER analysis, the structures reveal that ATP-powered isomerization entails changes in the relative symmetry of the BmrC and BmrD subunits that propagates from the transmembrane domain to the nucleotide binding domain. The structures uncover asymmetric substrate and Mg binding which we hypothesize are required for triggering ATP hydrolysis preferentially in one of the nucleotide-binding sites. MD simulations demonstrate that multiple lipid molecules differentially bind the IF versus the OC conformation thus establishing that lipid interactions modulate BmrCD energy landscape. Our findings are framed in a model that highlights the role of asymmetric conformations in the ATP-coupled transport with general implications to the mechanism of ABC transporters. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:37938578 / PubMed:37938578 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.9 - 5.65 Å |
| Structure data | EMDB-29087, PDB-8fhk: EMDB-29297: Structure0915 EMDB-29362, PDB-8fpf: EMDB-40908, PDB-8szc: EMDB-40974: BmrCD_OC-ADPVi EMDB-41004, PDB-8t3k:  EMDB-41058: Heterodimeric ABC transporter BmrCD in the inward-facing conformation bound to substrate and ADPVi: BmrCD_IF-HT/ADPVi |
| Chemicals |  ChemComp-ATP:  ChemComp-POV:  ChemComp-MG:  ChemComp-HT1: 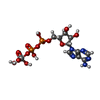 ChemComp-AOV: |
| Source |
|
 Keywords Keywords |  TRANSLOCASE / TRANSLOCASE /  transporter / transporter /  complex / complex /  lipids / lipids /  MEMBRANE PROTEIN / MEMBRANE PROTEIN /  Transport protein Transport protein |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers