+Search query
-Structure paper
| Title | The In Situ Structure of Parkinson's Disease-Linked LRRK2. |
|---|---|
| Journal, issue, pages | Cell, Vol. 182, Issue 6, Page 1508-1518.e16, Year 2020 |
| Publish date | Sep 17, 2020 |
 Authors Authors | Reika Watanabe / Robert Buschauer / Jan Böhning / Martina Audagnotto / Keren Lasker / Tsan-Wen Lu / Daniela Boassa / Susan Taylor / Elizabeth Villa /  |
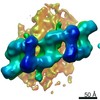
| PubMed Abstract | Mutations in leucine-rich repeat kinase 2 (LRRK2) are the most frequent cause of familial Parkinson's disease. LRRK2 is a multi-domain protein containing a kinase and GTPase. Using correlative light ...Mutations in leucine-rich repeat kinase 2 (LRRK2) are the most frequent cause of familial Parkinson's disease. LRRK2 is a multi-domain protein containing a kinase and GTPase. Using correlative light and electron microscopy, in situ cryo-electron tomography, and subtomogram analysis, we reveal a 14-Å structure of LRRK2 bearing a pathogenic mutation that oligomerizes as a right-handed double helix around microtubules, which are left-handed. Using integrative modeling, we determine the architecture of LRRK2, showing that the GTPase and kinase are in close proximity, with the GTPase closer to the microtubule surface, whereas the kinase is exposed to the cytoplasm. We identify two oligomerization interfaces mediated by non-catalytic domains. Mutation of one of these abolishes LRRK2 microtubule-association. Our work demonstrates the power of cryo-electron tomography to generate models of previously unsolved structures in their cellular environment. |
 External links External links |  Cell / Cell /  PubMed:32783917 / PubMed:32783917 /  PubMed Central PubMed Central |
| Methods | EM (subtomogram averaging) / EM (tomography) |
| Resolution | 14.0 - 18.0 Å |
| Structure data | EMDB-20825: In situ structure of LRRK2(I2020T)-Microtubule: Microtubule_bound_LRRK2(I2020T)_Tight Mask_A  EMDB-20826:  EMDB-20827:  EMDB-20828: |
| Source |
|
 Keywords Keywords |  SIGNALING PROTEIN / SIGNALING PROTEIN /  TRANSFERASE / TRANSFERASE /  kinase / kinase /  GTPase / GTPase /  Parkinson's Disease / pseudo-kinase Parkinson's Disease / pseudo-kinase |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers