+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3j07 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Model of a 24mer alphaB-crystallin multimer | ||||||
 Components Components | Alpha-crystallin B chain CRYAB CRYAB | ||||||
 Keywords Keywords |  CHAPERONE / sHSP CHAPERONE / sHSP | ||||||
| Function / homology |  Function and homology information Function and homology informationmicrotubule polymerization or depolymerization / negative regulation of intracellular transport / apoptotic process involved in morphogenesis / cardiac myofibril /  regulation of programmed cell death / tubulin complex assembly / structural constituent of eye lens / negative regulation of amyloid fibril formation / regulation of programmed cell death / tubulin complex assembly / structural constituent of eye lens / negative regulation of amyloid fibril formation /  M band / lens development in camera-type eye ...microtubule polymerization or depolymerization / negative regulation of intracellular transport / apoptotic process involved in morphogenesis / cardiac myofibril / M band / lens development in camera-type eye ...microtubule polymerization or depolymerization / negative regulation of intracellular transport / apoptotic process involved in morphogenesis / cardiac myofibril /  regulation of programmed cell death / tubulin complex assembly / structural constituent of eye lens / negative regulation of amyloid fibril formation / regulation of programmed cell death / tubulin complex assembly / structural constituent of eye lens / negative regulation of amyloid fibril formation /  M band / lens development in camera-type eye / muscle organ development / actin filament bundle / HSF1-dependent transactivation / negative regulation of reactive oxygen species metabolic process / negative regulation of protein-containing complex assembly / stress-activated MAPK cascade / M band / lens development in camera-type eye / muscle organ development / actin filament bundle / HSF1-dependent transactivation / negative regulation of reactive oxygen species metabolic process / negative regulation of protein-containing complex assembly / stress-activated MAPK cascade /  synaptic membrane / synaptic membrane /  muscle contraction / cellular response to gamma radiation / response to hydrogen peroxide / negative regulation of cell growth / Z disc / unfolded protein binding / muscle contraction / cellular response to gamma radiation / response to hydrogen peroxide / negative regulation of cell growth / Z disc / unfolded protein binding /  protein folding / response to estradiol / protein folding / response to estradiol /  amyloid-beta binding / response to heat / amyloid-beta binding / response to heat /  perikaryon / protein refolding / perikaryon / protein refolding /  microtubule binding / microtubule binding /  dendritic spine / dendritic spine /  lysosome / response to hypoxia / protein stabilization / lysosome / response to hypoxia / protein stabilization /  axon / negative regulation of gene expression / negative regulation of DNA-templated transcription / protein-containing complex binding / negative regulation of apoptotic process / structural molecule activity / axon / negative regulation of gene expression / negative regulation of DNA-templated transcription / protein-containing complex binding / negative regulation of apoptotic process / structural molecule activity /  cell surface / protein homodimerization activity / protein-containing complex / cell surface / protein homodimerization activity / protein-containing complex /  mitochondrion / extracellular exosome / mitochondrion / extracellular exosome /  nucleoplasm / identical protein binding / nucleoplasm / identical protein binding /  metal ion binding / metal ion binding /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | SOLID-STATE NMR /  SOLUTION SCATTERING / SOLUTION SCATTERING /  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  simulated annealing, simulated annealing,  molecular dynamics, torsion angle dynamics / molecular dynamics, torsion angle dynamics /  negative staining / Resolution: 20 Å negative staining / Resolution: 20 Å | ||||||
 Authors Authors | Jehle, S. / Vollmar, B. / Bardiaux, B. / Dove, K.K. / Rajagopal, P. / Gonen, T. / Oschkinat, H. / Klevit, R.E. | ||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2009 Journal: Proc Natl Acad Sci U S A / Year: 2009Title: The eye lens chaperone alpha-crystallin forms defined globular assemblies. Authors: Jirka Peschek / Nathalie Braun / Titus M Franzmann / Yannis Georgalis / Martin Haslbeck / Sevil Weinkauf / Johannes Buchner /  Abstract: Alpha-crystallins are molecular chaperones that protect vertebrate eye lens proteins from detrimental protein aggregation. alphaB-Crystallin, 1 of the 2 alpha-crystallin isoforms, is also associated ...Alpha-crystallins are molecular chaperones that protect vertebrate eye lens proteins from detrimental protein aggregation. alphaB-Crystallin, 1 of the 2 alpha-crystallin isoforms, is also associated with myopathies and neuropathological diseases. Despite the importance of alpha-crystallins in protein homeostasis, only little is known about their quaternary structures because of their seemingly polydisperse nature. Here, we analyzed the structures of recombinant alpha-crystallins using biophysical methods. In contrast to previous reports, we show that alphaB-crystallin assembles into defined oligomers consisting of 24 subunits. The 3-dimensional (3D) reconstruction of alphaB-crystallin by electron microscopy reveals a sphere-like structure with large openings to the interior of the protein. alphaA-Crystallin forms, in addition to complexes of 24 subunits, also smaller oligomers and large clusters consisting of individual oligomers. This propensity might explain the previously reported polydisperse nature of alpha-crystallin. #1:  Journal: Nat Struct Mol Biol / Year: 2010 Journal: Nat Struct Mol Biol / Year: 2010Title: Solid-state NMR and SAXS studies provide a structural basis for the activation of alphaB-crystallin oligomers. Authors: Stefan Jehle / Ponni Rajagopal / Benjamin Bardiaux / Stefan Markovic / Ronald Kühne / Joseph R Stout / Victoria A Higman / Rachel E Klevit / Barth-Jan van Rossum / Hartmut Oschkinat /  Abstract: The small heat shock protein alphaB-crystallin (alphaB) contributes to cellular protection against stress. For decades, high-resolution structural studies on oligomeric alphaB have been confounded by ...The small heat shock protein alphaB-crystallin (alphaB) contributes to cellular protection against stress. For decades, high-resolution structural studies on oligomeric alphaB have been confounded by its polydisperse nature. Here, we present a structural basis of oligomer assembly and activation of the chaperone using solid-state NMR and small-angle X-ray scattering (SAXS). The basic building block is a curved dimer, with an angle of approximately 121 degrees between the planes of the beta-sandwich formed by alpha-crystallin domains. The highly conserved IXI motif covers a substrate binding site at pH 7.5. We observe a pH-dependent modulation of the interaction of the IXI motif with beta4 and beta8, consistent with a pH-dependent regulation of the chaperone function. N-terminal region residues Ser59-Trp60-Phe61 are involved in intermolecular interaction with beta3. Intermolecular restraints from NMR and volumetric restraints from SAXS were combined to calculate a model of a 24-subunit alphaB oligomer with tetrahedral symmetry. | ||||||
| History |
| ||||||
| Remark 0 | THIS ENTRY 3J07 CONTAINS A STRUCTURAL MODEL FIT TO AN ELECTRON MICROSCOPY MAP (EMD-1776) DETERMINED ...THIS ENTRY 3J07 CONTAINS A STRUCTURAL MODEL FIT TO AN ELECTRON MICROSCOPY MAP (EMD-1776) DETERMINED ORIGINALLY BY AUTHORS: J.PESCHEK, N.BRAUN, T.M.FRANZMANN, Y.GEORGALIS, M.HASLBECK, S.WEINKAUF, J.BUCHNER |
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3j07.cif.gz 3j07.cif.gz | 870.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3j07.ent.gz pdb3j07.ent.gz | 735.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3j07.json.gz 3j07.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/j0/3j07 https://data.pdbj.org/pub/pdb/validation_reports/j0/3j07 ftp://data.pdbj.org/pub/pdb/validation_reports/j0/3j07 ftp://data.pdbj.org/pub/pdb/validation_reports/j0/3j07 | HTTPS FTP |
|---|
-Related structure data
| Related structure data | 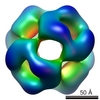 1776M M: map data used to model this data |
|---|---|
| Similar structure data | |
| Other databases |
|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein |  CRYAB / Alpha(B)-crystallin / Heat shock protein beta-5 / HspB5 / Renal carcinoma antigen NY-REN-27 / ...Alpha(B)-crystallin / Heat shock protein beta-5 / HspB5 / Renal carcinoma antigen NY-REN-27 / Rosenthal fiber component CRYAB / Alpha(B)-crystallin / Heat shock protein beta-5 / HspB5 / Renal carcinoma antigen NY-REN-27 / ...Alpha(B)-crystallin / Heat shock protein beta-5 / HspB5 / Renal carcinoma antigen NY-REN-27 / Rosenthal fiber componentMass: 20191.930 Da / Num. of mol.: 24 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: CRYAB, CRYA2 / Plasmid: pET16b / Production host: Homo sapiens (human) / Gene: CRYAB, CRYA2 / Plasmid: pET16b / Production host:   Escherichia coli (E. coli) / References: UniProt: P02511 Escherichia coli (E. coli) / References: UniProt: P02511 |
|---|
-Experimental details
-Experiment
| Experiment |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR experiment |
|
- Sample preparation
Sample preparation
| Component | Name: 24mer alphaB-crystallin multimer / Type: COMPLEX / Details: polydisperse oligomer | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer solution | pH: 7.4 / Details: 137mM NaCl, 2.7mM KCl, 12 mM PBS | ||||||||||||||||||||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : YES / Vitrification applied : YES / Vitrification applied : NO : NO | ||||||||||||||||||||||||||||||||||||
| EM staining | Type: NEGATIVE / Material: Uranyl Acetate | ||||||||||||||||||||||||||||||||||||
| Crystal | Description: HOMOLOGY MODELS AND SPARSE SOLID-STATE NMR DISTANCE RESTRAINTS WERE USED TO DEFINE THE SECONDARY STRUCTURE OF THE N-TERMINAL DOMAIN (UNP RESIDUES 1-65). THE RESTRAINTS ARE GIVEN IN THE ...Description: HOMOLOGY MODELS AND SPARSE SOLID-STATE NMR DISTANCE RESTRAINTS WERE USED TO DEFINE THE SECONDARY STRUCTURE OF THE N-TERMINAL DOMAIN (UNP RESIDUES 1-65). THE RESTRAINTS ARE GIVEN IN THE SUPPORTING INFORMATION OF THE PRIMARY CITATION. A 2D 1H-13C INEPT SPECTRUM WAS RECORDED AND ANALYZED IN ORDER TO IDENTIFY FLEXIBLE RESIDUES IN THE EXTREME N- AND C-TERMINI. SEVERAL MODELS WERE PREPARED WITH DIFFERENT POSITIONS OF FLEXIBLE RESIDUES IN THE INSIDE OF THE SPHERICAL MULTIMER TO FIT THE RADIUS OF GYRATION (RG) MEASURED BY SAXS. THE MODEL THAT BEST FIT THE RG WAS CHOSEN FOR DEPOSITION. | ||||||||||||||||||||||||||||||||||||
| Details |
| ||||||||||||||||||||||||||||||||||||
| Sample |
| ||||||||||||||||||||||||||||||||||||
| Sample conditions | pH: 7.6 / Temperature: 270 K |
-Data collection
| Microscopy | Model: JEOL 100CX / Date: Feb 6, 2008 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Electron gun | Electron source : OTHER / Accelerating voltage: 100 kV / Illumination mode: FLOOD BEAM : OTHER / Accelerating voltage: 100 kV / Illumination mode: FLOOD BEAM | |||||||||||||||||||||||||
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal magnification: 50000 X / Nominal defocus max: 1200 nm / Nominal defocus min: 900 nm Bright-field microscopy / Nominal magnification: 50000 X / Nominal defocus max: 1200 nm / Nominal defocus min: 900 nm | |||||||||||||||||||||||||
| Image recording | Film or detector model: KODAK SO-163 FILM | |||||||||||||||||||||||||
| NMR spectrometer |
| |||||||||||||||||||||||||
| Soln scatter | Type: x-ray / Buffer name: phosphate, 100 mM NaCl / Conc. range: 0.1-12.6 mg/mL / Data reduction software list: CBF-PRIMUS / Detector type: 2D MAR345 / Mean guiner radius: 5.6 nm / Mean guiner radius esd: 0.2 nm / Num. of time frames: 1 / Protein length: 14.5 / Sample pH: 7.5 / Source beamline: X33 / Source class: Y / Source type: DESY HAMBURG X33 BEAMLINE / Temperature: 293 K |
- Processing
Processing
| Symmetry | Point symmetry : T (tetrahedral : T (tetrahedral ) ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
3D reconstruction | Resolution: 20 Å / Resolution method: FSC 0.5 CUT-OFF / Num. of particles: 2565 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 2KLR | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | Method:  simulated annealing, simulated annealing,  molecular dynamics, torsion angle dynamics molecular dynamics, torsion angle dynamicsSoftware ordinal: 11 Details: THREE DIMERS CONNECTED BY THEIR C-TERMINAL IXI MOTIF WERE FITTED BACK-TO-BACK IN THE EM DENSITY USING CHIMERA. A HEXAMER WAS BUILT FROM MODEL #7 OF PDB ENTRY 2KLR USING NONCRYSTALLOGRAPHIC ...Details: THREE DIMERS CONNECTED BY THEIR C-TERMINAL IXI MOTIF WERE FITTED BACK-TO-BACK IN THE EM DENSITY USING CHIMERA. A HEXAMER WAS BUILT FROM MODEL #7 OF PDB ENTRY 2KLR USING NONCRYSTALLOGRAPHIC SYMMETRY RESTRAINTS GIVEN IN THE PDB FILE. DIMERS WERE CONNECTED BY THEIR C-TERMINAL DOMAIN CONTAINING THE IXI-MOTIF, AND THE LINKER (UNP RESIDUES 151-155) WAS ENERGY MINIMIZED USING XPLOR-NIH. THE BETA1/BETA2(FREE) STRUCTURAL SEGMENT WAS PLACED INTO THE EM DENSITY TO FULFILL THE CROSSLINKS FOR A57, S59, AND T63, SIMILAR TO PDB ENTRY 1VLQ. ALPHA2 HELICES OF TWO MONOMERS WERE DOCKED, DEFINING RESIDUES L32, L33, L37, AND F38 AS INTERACTING RESIDUES USING HADDOCK. THE ALPHA2 HELICAL COMPLEX WAS PLACED IN WEAK EM DENSITY AT THE THREE-FOLD AXIS. THE FLEXIBLE C- AND N-TERMINAL RESIDUES, INCLUDING PARTS OF HELIX ALPHA1, WERE PLACED INSIDE THE 24MER TO FIT THE RADIUS OF GYRATION, MEASURED BY SMALL-ANGLE X-RAY SCATTERING IN AN ITERATIVE PROCESS. ALL FRAGMENTS WERE CONNECTED USING DISCOVERY STUDIO 1.6 (ACCELRYS) AND THE LINKERS WERE ENERGY MINIMIZED. BACK PROJECTIONS WERE CALCULATED FOR MODELS OF ALPHAB MULTIMERS CONTAINING 24, 26, AND 28 SUBUNITS USING THE PROGRAM SPIDER. THE CALCULATED PROJECTIONS ARE SIMILAR TO EACH OTHER, TO THE CLASS SUM IMAGES FROM COLLECTED EM DATA, AND TO THOSE CALCULATED FROM THE PUBLISHED EM DENSITY (EMD-1776). DETAILS ARE GIVEN IN THE PRIMARY CITATION. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR representative | Selection criteria: lowest energy | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: structures with the lowest energy Conformers calculated total number: 200 / Conformers submitted total number: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Soln scatter model | Method: FITTING OF MODELS WITH SAXS DATA; Conformer selection criteria: BEST FIT WITH NMR, SAXS, AND EM DATA Details: TO DETERMINE HETEROGENEITY, A PYTHON SCRIPT WAS USED TO MODULATE AND FIT THE THEORETICAL SAXS CURVE OF THE MODEL WITH THE EXPERIMENTAL DATA. THE ALGORITHM THAT WAS APPLIED HAS BEEN PUBLISHED ...Details: TO DETERMINE HETEROGENEITY, A PYTHON SCRIPT WAS USED TO MODULATE AND FIT THE THEORETICAL SAXS CURVE OF THE MODEL WITH THE EXPERIMENTAL DATA. THE ALGORITHM THAT WAS APPLIED HAS BEEN PUBLISHED BY MITTELBACH, R. AND GLATTER, O. (1998) IN J. APPL. CRYSTALLOGR. 31:600-608. FOR DETAILS SEE PRIMARY CITATION. Num. of conformers calculated: 10 / Num. of conformers submitted: 1 / Representative conformer: 1 Software author list: PETOUKHOV, M.V.; SVERGUN, D.I.; SCHWIETERS, C.D. PETTERSON, E.F.; GODDARD, T.D.; ACCELRYS Software list: CHIMERA, PRIMUS, GNOM, XPLOR-NIH, CRYSOL, DISCOVERY STUDIO 1.3; |
 Movie
Movie Controller
Controller









 PDBj
PDBj


