[English] 日本語
 Yorodumi
Yorodumi- EMDB-4266: ACP2 crosslinked to the KS of the loading/condensing region of th... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4266 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | ACP2 crosslinked to the KS of the loading/condensing region of the CTB1 PKS | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology information phosphopantetheine binding / 3-oxoacyl-[acyl-carrier-protein] synthase activity / phosphopantetheine binding / 3-oxoacyl-[acyl-carrier-protein] synthase activity /  Transferases; Acyltransferases; Transferring groups other than aminoacyl groups / fatty acid biosynthetic process Transferases; Acyltransferases; Transferring groups other than aminoacyl groups / fatty acid biosynthetic processSimilarity search - Function | ||||||||||||||||||
| Biological species |   Cercospora nicotianae (fungus) Cercospora nicotianae (fungus) | ||||||||||||||||||
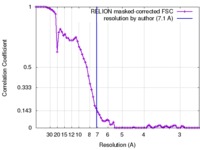
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 7.1 Å cryo EM / Resolution: 7.1 Å | ||||||||||||||||||
 Authors Authors | Herbst DA / Huitt-Roehl CR / Jakob RP / Townsend CA / Maier T | ||||||||||||||||||
| Funding support |  Switzerland, Switzerland,  United States, 5 items United States, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Chem Biol / Year: 2018 Journal: Nat Chem Biol / Year: 2018Title: The structural organization of substrate loading in iterative polyketide synthases. Authors: Dominik A Herbst / Callie R Huitt-Roehl / Roman P Jakob / Jacob M Kravetz / Philip A Storm / Jamie R Alley / Craig A Townsend / Timm Maier /   Abstract: Polyketide synthases (PKSs) are microbial multienzymes for the biosynthesis of biologically potent secondary metabolites. Polyketide production is initiated by the loading of a starter unit onto an ...Polyketide synthases (PKSs) are microbial multienzymes for the biosynthesis of biologically potent secondary metabolites. Polyketide production is initiated by the loading of a starter unit onto an integral acyl carrier protein (ACP) and its subsequent transfer to the ketosynthase (KS). Initial substrate loading is achieved either by multidomain loading modules or by the integration of designated loading domains, such as starter unit acyltransferases (SAT), whose structural integration into PKS remains unresolved. A crystal structure of the loading/condensing region of the nonreducing PKS CTB1 demonstrates the ordered insertion of a pseudodimeric SAT into the condensing region, which is aided by the SAT-KS linker. Cryo-electron microscopy of the post-loading state trapped by mechanism-based crosslinking of ACP to KS reveals asymmetry across the CTB1 loading/-condensing region, in accord with preferential 1:2 binding stoichiometry. These results are critical for re-engineering the loading step in polyketide biosynthesis and support functional relevance of asymmetric conformations of PKSs. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4266.map.gz emd_4266.map.gz | 32.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4266-v30.xml emd-4266-v30.xml emd-4266.xml emd-4266.xml | 16.9 KB 16.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4266_fsc.xml emd_4266_fsc.xml | 7.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_4266.png emd_4266.png | 46 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4266 http://ftp.pdbj.org/pub/emdb/structures/EMD-4266 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4266 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4266 | HTTPS FTP |
-Related structure data
| Related structure data |  6fikMC  6fijC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4266.map.gz / Format: CCP4 / Size: 35.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4266.map.gz / Format: CCP4 / Size: 35.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.326 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : CTB1-SAT0-KS-MAT0=ACP2
| Entire | Name: CTB1-SAT0-KS-MAT0=ACP2 |
|---|---|
| Components |
|
-Supramolecule #1: CTB1-SAT0-KS-MAT0=ACP2
| Supramolecule | Name: CTB1-SAT0-KS-MAT0=ACP2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Cercospora nicotianae (fungus) Cercospora nicotianae (fungus) |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
-Macromolecule #1: Polyketide synthase
| Macromolecule | Name: Polyketide synthase / type: protein_or_peptide / ID: 1 / Details: C553 in chain A is crosslinked to S1816 in chain C / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Cercospora nicotianae (fungus) Cercospora nicotianae (fungus) |
| Molecular weight | Theoretical: 140.255 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MEDGAQMRVV AFGDQTYDCS EAVSQLLRVR DDAIVVDFLE RAPAVLKAEL ARLSSEQQEE TPRFATLAEL VPRYRAGTLN PAVSQALTC IAQLGLFIRQ HSSGQEAYPT AHDSCITGVA TGALTAVAVG SASSVTALVP LALHTVAVAV RLGARAWEIG S CLADARRG ...String: MEDGAQMRVV AFGDQTYDCS EAVSQLLRVR DDAIVVDFLE RAPAVLKAEL ARLSSEQQEE TPRFATLAEL VPRYRAGTLN PAVSQALTC IAQLGLFIRQ HSSGQEAYPT AHDSCITGVA TGALTAVAVG SASSVTALVP LALHTVAVAV RLGARAWEIG S CLADARRG ANGRYASWTS AVGGISPQDL QDRISAYTAE QALASVSVPY LSAAVGPGQS SVSAAPVILD AFLSTLLRPL TT TRLPITA PYHAPHLFTA KDVQHVTDCL PPSEAWPTVR IPIISFSRDE AVSRGASFPA AMSEAVRDCL IRPIALDRMA VSI ANHARD LGKDSVLPSP IALSFSDKLG PQVNSHLPGA KAPTPELTSK SIPSAIGAEQ QPMAKSPIAI LAASGRFPQS SSMD QFWDV LINGVDTHEL VPPTRWNAAT HVSEDPKAKN VSGTGFGCWL HEAGEFDAAY FNMSPREAPQ VDPAQRLALL TATEA LEQA GVVPNRTSST QKNRVGVWYG ATSNDWMETN SAQNVDTYFI PGGNRAFIPG RVNYFHKFSG PSYTIDTACS SSLAAL HMA CNALWRGEVD TAIVGGTNVL TNPDMTAGLD AGHFLSRSGN CKTFDDEADG YCRGEAVVTL ILKRLPDAQA DKDPIQA SI LGIATNHSAE AASITRPHAG AQQDLFQQVL TETGLTANDI SVCEMHGTGT QAGDSGETTS VVETLAPLNR SGSAVRTT P LYIGAVKSNV GHAESAAGVS SLAKILLMLK HSKIPPHVGI KTKLNHRLPD LAARNTHIAR SEVPWPRPKN GKRRVLLNN FSAAGGNTCL VLEDAPEPED SQEVDPREHH IVALSAKTPD SMVNNLTNMI TWIDKHSGDS LATLPQLSYT TTARRVHHRH RAVATGTDL LQIRSSLQEQ LDRRVSGERS IPHPPNGPSF VLAFTGQGSA FAGMGVDLYK RFASFRSDIA RYDQICEGMS L PSIKAMFE DEKVFSTASP TLQQLTHVCF QMALYRLWKS LGVQAKAVVG HALGEYAALY AAGVLSQSDT LYLVGRRAQL ME KHLSQGT HAMLAVRAKE EAIVAAIDGP PGEAYEFSCR NGEQRNVLGG TVAQIQAAKA ALEAKKIRCQ YLDTPMAFHT GQV DPILPE LLQVAAACSI QDPQIPVISP AYGKVIRSAK DFQPEYFTHH CRSSVNMVDA LQSAVEEGLL DKNVIGLEIG PGPV VTQFV KEAVGTTMQT FASINKDKDT WQLMTQALAK FYLAGASVEW SRYHEDFPGA QKVLELPAYG WALKNYWLQY VNDWS LRKG DPAVVVAASA AALEHHHHHH |
-Macromolecule #2: Polyketide synthase
| Macromolecule | Name: Polyketide synthase / type: protein_or_peptide / ID: 2 / Details: S1816 is crosslinked to C553 in chain A / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Cercospora nicotianae (fungus) Cercospora nicotianae (fungus) |
| Molecular weight | Theoretical: 9.892837 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: GSHMDPSPNE IGTVWRDALK ILSEESGLTD EELTDDTSFA DVGVDSLMSL VITSRLRDEL DIDFPDRALF EECQTIFDLR KRFSGSTE |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.27 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| ||||||||||||
| Grid | Model: Quantifoil Lacey carbon grid / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: LACEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK III | ||||||||||||
| Details | The proteins have been crosslinked via site specific crosslinking. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 4.5 µm / Calibrated defocus min: 0.8 µm / Calibrated magnification: 27707 / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 0.8 µm / Nominal magnification: 105000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 0.8 µm / Nominal magnification: 105000 |
| Specialist optics | Energy filter - Lower energy threshold: 0 eV / Energy filter - Upper energy threshold: 20 eV |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 41.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller