+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13063 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The structure of MutS bound to two molecules of AMPPNP | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationadenine/cytosine mispair binding /  MutS complex / MutS complex /  mismatch repair complex / regulation of DNA recombination / mismatched DNA binding / DNA binding, bending / ATP-dependent DNA damage sensor activity / mismatch repair complex / regulation of DNA recombination / mismatched DNA binding / DNA binding, bending / ATP-dependent DNA damage sensor activity /  mismatch repair / mismatch repair /  ADP binding / damaged DNA binding ...adenine/cytosine mispair binding / ADP binding / damaged DNA binding ...adenine/cytosine mispair binding /  MutS complex / MutS complex /  mismatch repair complex / regulation of DNA recombination / mismatched DNA binding / DNA binding, bending / ATP-dependent DNA damage sensor activity / mismatch repair complex / regulation of DNA recombination / mismatched DNA binding / DNA binding, bending / ATP-dependent DNA damage sensor activity /  mismatch repair / mismatch repair /  ADP binding / damaged DNA binding / DNA damage response / ADP binding / damaged DNA binding / DNA damage response /  ATP hydrolysis activity / ATP hydrolysis activity /  ATP binding / identical protein binding / ATP binding / identical protein binding /  cytosol cytosolSimilarity search - Function | |||||||||
| Biological species |   Escherichia coli (E. coli) / Escherichia coli (E. coli) /   Escherichia coli (strain K12) (bacteria) Escherichia coli (strain K12) (bacteria) | |||||||||
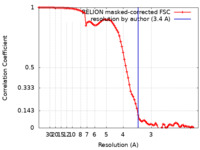
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.4 Å cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Lamers MH / Borsellini A / Friedhoff P / Kunetsky V | |||||||||
| Funding support | European Union, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2022 Journal: Nat Struct Mol Biol / Year: 2022Title: Cryogenic electron microscopy structures reveal how ATP and DNA binding in MutS coordinates sequential steps of DNA mismatch repair. Authors: Alessandro Borsellini / Vladislav Kunetsky / Peter Friedhoff / Meindert H Lamers /   Abstract: DNA mismatch repair detects and corrects mismatches introduced during DNA replication. The protein MutS scans for mismatches and coordinates the repair cascade. During this process, MutS undergoes ...DNA mismatch repair detects and corrects mismatches introduced during DNA replication. The protein MutS scans for mismatches and coordinates the repair cascade. During this process, MutS undergoes multiple conformational changes in response to ATP binding, hydrolysis and release, but how ATP induces the various MutS conformations is incompletely understood. Here we present four cryogenic electron microscopy structures of Escherichia coli MutS at sequential stages of the ATP hydrolysis cycle that reveal how ATP binding and hydrolysis induce closing and opening of the MutS dimer, respectively. Biophysical analysis demonstrates how DNA binding modulates the ATPase cycle by prevention of hydrolysis during scanning and mismatch binding, while preventing ADP release in the sliding clamp state. Nucleotide release is achieved when MutS encounters single-stranded DNA that is produced during removal of the daughter strand. The combination of ATP binding and hydrolysis and its modulation by DNA enables MutS to adopt the different conformations needed to coordinate the sequential steps of the mismatch repair cascade. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13063.map.gz emd_13063.map.gz | 2.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13063-v30.xml emd-13063-v30.xml emd-13063.xml emd-13063.xml | 14.6 KB 14.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13063_fsc.xml emd_13063_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_13063.png emd_13063.png | 146.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13063 http://ftp.pdbj.org/pub/emdb/structures/EMD-13063 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13063 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13063 | HTTPS FTP |
-Related structure data
| Related structure data |  7otoMC  7ou0C  7ou2C  7ou4C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_13063.map.gz / Format: CCP4 / Size: 7.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13063.map.gz / Format: CCP4 / Size: 7.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : DNA mismatch repair protein MutS
| Entire | Name: DNA mismatch repair protein MutS |
|---|---|
| Components |
|
-Supramolecule #1: DNA mismatch repair protein MutS
| Supramolecule | Name: DNA mismatch repair protein MutS / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 / Details: MutS bound to two molecules of AMPPNP |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Molecular weight | Theoretical: 190 KDa |
| Recombinant expression | Organism:   Escherichia coli 'BL21-Gold(DE3)pLysS AG' (bacteria) Escherichia coli 'BL21-Gold(DE3)pLysS AG' (bacteria) |
-Macromolecule #1: DNA mismatch repair protein MutS
| Macromolecule | Name: DNA mismatch repair protein MutS / type: protein_or_peptide / ID: 1 / Details: AMPPNP / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Escherichia coli (strain K12) (bacteria) / Strain: K12 Escherichia coli (strain K12) (bacteria) / Strain: K12 |
| Molecular weight | Theoretical: 90.433234 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: HHHHHHMSAI ENFDAHTPMM QQYLRLKAQH PEILLFYRMG DFYELFYDDA KRASQLLDIS LTKRGASAGE PIPMAGIPYH AVENYLAKL VNQGESVAIC EQIGDPATSK GPVERKVVRI VTPGTISDEA LLQERQDNLL AAIWQDSKGF GYATLDISSG R FRLSEPAD ...String: HHHHHHMSAI ENFDAHTPMM QQYLRLKAQH PEILLFYRMG DFYELFYDDA KRASQLLDIS LTKRGASAGE PIPMAGIPYH AVENYLAKL VNQGESVAIC EQIGDPATSK GPVERKVVRI VTPGTISDEA LLQERQDNLL AAIWQDSKGF GYATLDISSG R FRLSEPAD RETMAAELQR TNPAELLYAE DFAEMSLIEG RRGLRRRPLW EFEIDTARQQ LNLQFGTRDL VGFGVENAPR GL CAAGCLL QYAKDTQRTT LPHIRSITME REQDSIIMDA ATRRNLEITQ NLAGGAENTL ASVLDCTVTP MGSRMLKRWL HMP VRDTRV LLERQQTIGA LQDFTAGLQP VLRQVGDLER ILARLALRTA RPRDLARMRH AFQQLPELRA QLETVDSAPV QALR EKMGE FAELRDLLER AIIDTPPVLV RDGGVIASGY NEELDEWRAL ADGATDYLER LEVRERERTG LDTLKVGFNA VHGYY IQIS RGQSHLAPIN YMRRQTLKNA ERYIIPELKE YEDKVLTSKG KALALEKQLY EELFDLLLPH LEALQQSASA LAELDV LVN LAERAYTLNY TCPTFIDKPG IRITEGRHPV VEQVLNEPFI ANPLNLSPQR RMLIITGPNM GGKSTYMRQT ALIALMA YI GSYVPAQKVE IGPIDRIFTR VGAADDLASG RSTFMVEMTE TANILHNATE YSLVLMDEIG RGTSTYDGLS LAWACAEN L ANKIKALTLF ATHYFELTQL PEKMEGVANV HLDALEHGDT IAFMHSVQDG AASKSYGLAV AALAGVPKEV IKRARQKLR ELESIS |
-Macromolecule #2: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 2 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #3: PHOSPHOAMINOPHOSPHONIC ACID-ADENYLATE ESTER
| Macromolecule | Name: PHOSPHOAMINOPHOSPHONIC ACID-ADENYLATE ESTER / type: ligand / ID: 3 / Number of copies: 2 / Formula: ANP |
|---|---|
| Molecular weight | Theoretical: 506.196 Da |
| Chemical component information |  ChemComp-ANP: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.95 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8.5 Component:
| ||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.0004 kPa | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 130000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 130000 |
| Specialist optics | Energy filter - Slit width: 20 eV |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 57.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller