+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1ak2 | ||||||
|---|---|---|---|---|---|---|---|
| Title | ADENYLATE KINASE ISOENZYME-2 | ||||||
 Components Components | ADENYLATE KINASE ISOENZYME-2 | ||||||
 Keywords Keywords |  PHOSPHOTRANSFERASE / NUCLEOSIDE MONOPHOSPHATE KINASE PHOSPHOTRANSFERASE / NUCLEOSIDE MONOPHOSPHATE KINASE | ||||||
| Function / homology |  Function and homology information Function and homology informationInterconversion of nucleotide di- and triphosphates / AMP metabolic process / ADP biosynthetic process /  adenylate kinase / adenylate kinase /  adenylate kinase activity / sperm mitochondrial sheath / ATP metabolic process / adenylate kinase activity / sperm mitochondrial sheath / ATP metabolic process /  mitochondrial intermembrane space / mitochondrial intermembrane space /  mitochondrial inner membrane / mitochondrial inner membrane /  phosphorylation ...Interconversion of nucleotide di- and triphosphates / AMP metabolic process / ADP biosynthetic process / phosphorylation ...Interconversion of nucleotide di- and triphosphates / AMP metabolic process / ADP biosynthetic process /  adenylate kinase / adenylate kinase /  adenylate kinase activity / sperm mitochondrial sheath / ATP metabolic process / adenylate kinase activity / sperm mitochondrial sheath / ATP metabolic process /  mitochondrial intermembrane space / mitochondrial intermembrane space /  mitochondrial inner membrane / mitochondrial inner membrane /  phosphorylation / phosphorylation /  mitochondrion / mitochondrion /  ATP binding / ATP binding /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Bos taurus (cattle) Bos taurus (cattle) | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 1.92 Å X-RAY DIFFRACTION / Resolution: 1.92 Å | ||||||
 Authors Authors | Schlauderer, G.J. / Schulz, G.E. | ||||||
 Citation Citation |  Journal: Protein Sci. / Year: 1996 Journal: Protein Sci. / Year: 1996Title: The structure of bovine mitochondrial adenylate kinase: comparison with isoenzymes in other compartments. Authors: Schlauderer, G.J. / Schulz, G.E. #1:  Journal: Structure / Year: 1995 Journal: Structure / Year: 1995Title: Movie of the Structural Changes During a Catalytic Cycle of Nucleoside Monophosphate Kinases Authors: Vonrhein, C. / Schlauderer, G.J. / Schulz, G.E. #2:  Journal: J.Biol.Chem. / Year: 1987 Journal: J.Biol.Chem. / Year: 1987Title: Isolation and Characterization of Two Types of Cdna for Mitochondrial Adenylate Kinase and Their Expression in Escherichia Coli Authors: Kishi, F. / Tanizawa, Y. / Nakazawa, A. #3:  Journal: Eur.J.Biochem. / Year: 1986 Journal: Eur.J.Biochem. / Year: 1986Title: Mitochondrial Adenylate Kinase (Ak2) from Bovine Heart. The Complete Primary Structure Authors: Frank, R. / Trosin, M. / Tomasselli, A.G. / Noda, L. / Krauth-Siegel, R.L. / Schirmer, R.H. #4:  Journal: Eur.J.Biochem. / Year: 1980 Journal: Eur.J.Biochem. / Year: 1980Title: Mitochondrial ATP:AMP Phosphotransferase from Beef Heart: Purification and Properties Authors: Tomasselli, A.G. / Noda, L.H. | ||||||
| History |
|
- Structure visualization
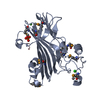
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1ak2.cif.gz 1ak2.cif.gz | 53 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1ak2.ent.gz pdb1ak2.ent.gz | 41.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1ak2.json.gz 1ak2.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ak/1ak2 https://data.pdbj.org/pub/pdb/validation_reports/ak/1ak2 ftp://data.pdbj.org/pub/pdb/validation_reports/ak/1ak2 ftp://data.pdbj.org/pub/pdb/validation_reports/ak/1ak2 | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 25540.504 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: PRECIPITANT POLYETHYLENE GLYCOL / Source: (natural)   Bos taurus (cattle) / Organ: LIVER Bos taurus (cattle) / Organ: LIVER / Organelle: MITOCHONDRIA INTERMEMBRANE SPACE / References: UniProt: P08166, / Organelle: MITOCHONDRIA INTERMEMBRANE SPACE / References: UniProt: P08166,  adenylate kinase adenylate kinase |
|---|---|
| #2: Chemical | ChemComp-SO4 /  Sulfate Sulfate |
| #3: Water | ChemComp-HOH /  Water Water |
| Compound details | THE SECONDARY STRUCTURAL ELEMENTS PRESENTED BELOW ARE FROM PROGRAM DSSP. FOR MANUAL ASSIGNMENTS ...THE SECONDARY STRUCTURAL |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.65 Å3/Da / Density % sol: 54 % |
|---|---|
Crystal grow | *PLUS Method: unknown |
| Components of the solutions | *PLUS Common name: PEG / Details: precipitant |
-Data collection
| Diffraction source | Source:  ROTATING ANODE / Wavelength: 1.5418 ROTATING ANODE / Wavelength: 1.5418 |
|---|---|
| Detector | Type: SIEMENS-NICOLET X100 / Detector: AREA DETECTOR / Date: 1992 |
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.5418 Å / Relative weight: 1 : 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.92→10 Å / Num. obs: 20850 / % possible obs: 98 % / Observed criterion σ(I): 0 / Redundancy: 13 % / Rmerge(I) obs: 0.077 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.92→10 Å / σ(F): 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 34 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.27 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.92→10 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Classification: refinement X-PLOR / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS |
 Movie
Movie Controller
Controller













 PDBj
PDBj


