[English] 日本語
 Yorodumi
Yorodumi- PDB-6qxu: Human TNKS1 in complex with 6,8-Difluoro-2-[4-(1-hydroxy-1-methyl... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6qxu | ||||||
|---|---|---|---|---|---|---|---|
| Title | Human TNKS1 in complex with 6,8-Difluoro-2-[4-(1-hydroxy-1-methyl-ethyl)-phenyl]-3H-quinazolin-4-one | ||||||
 Components Components | Poly [ADP-ribose] polymerase tankyrase-1 | ||||||
 Keywords Keywords | TRANSFERASE/TRANSFERASE INHIBITOR /  TANKYRASE / PARP / TANKYRASE / PARP /  INHIBITOR / TRANSFERASE-TRANSFERASE INHIBITOR COMPLEX / INHIBITOR / TRANSFERASE-TRANSFERASE INHIBITOR COMPLEX /  TRANSFERASE TRANSFERASE | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of telomeric DNA binding / negative regulation of maintenance of mitotic sister chromatid cohesion, telomeric / regulation of telomere maintenance via telomerase / XAV939 stabilizes AXIN /  NAD+ ADP-ribosyltransferase / protein localization to chromosome, telomeric region / negative regulation of telomere maintenance via telomere lengthening / protein auto-ADP-ribosylation / protein poly-ADP-ribosylation / NAD+ ADP-ribosyltransferase / protein localization to chromosome, telomeric region / negative regulation of telomere maintenance via telomere lengthening / protein auto-ADP-ribosylation / protein poly-ADP-ribosylation /  pericentriolar material ...negative regulation of telomeric DNA binding / negative regulation of maintenance of mitotic sister chromatid cohesion, telomeric / regulation of telomere maintenance via telomerase / XAV939 stabilizes AXIN / pericentriolar material ...negative regulation of telomeric DNA binding / negative regulation of maintenance of mitotic sister chromatid cohesion, telomeric / regulation of telomere maintenance via telomerase / XAV939 stabilizes AXIN /  NAD+ ADP-ribosyltransferase / protein localization to chromosome, telomeric region / negative regulation of telomere maintenance via telomere lengthening / protein auto-ADP-ribosylation / protein poly-ADP-ribosylation / NAD+ ADP-ribosyltransferase / protein localization to chromosome, telomeric region / negative regulation of telomere maintenance via telomere lengthening / protein auto-ADP-ribosylation / protein poly-ADP-ribosylation /  pericentriolar material / mitotic spindle pole / NAD+-protein ADP-ribosyltransferase activity / positive regulation of telomere capping / pericentriolar material / mitotic spindle pole / NAD+-protein ADP-ribosyltransferase activity / positive regulation of telomere capping /  NAD+ ADP-ribosyltransferase activity / NAD+ ADP-ribosyltransferase activity /  Transferases; Glycosyltransferases; Pentosyltransferases / mRNA transport / spindle assembly / Transferases; Glycosyltransferases; Pentosyltransferases / mRNA transport / spindle assembly /  nuclear pore / positive regulation of telomerase activity / positive regulation of telomere maintenance via telomerase / nuclear pore / positive regulation of telomerase activity / positive regulation of telomere maintenance via telomerase /  nucleotidyltransferase activity / mitotic spindle organization / TCF dependent signaling in response to WNT / Degradation of AXIN / peptidyl-threonine phosphorylation / nucleotidyltransferase activity / mitotic spindle organization / TCF dependent signaling in response to WNT / Degradation of AXIN / peptidyl-threonine phosphorylation /  Wnt signaling pathway / protein polyubiquitination / Regulation of PTEN stability and activity / positive regulation of canonical Wnt signaling pathway / Wnt signaling pathway / protein polyubiquitination / Regulation of PTEN stability and activity / positive regulation of canonical Wnt signaling pathway /  protein transport / protein transport /  histone binding / peptidyl-serine phosphorylation / histone binding / peptidyl-serine phosphorylation /  nuclear membrane / nuclear membrane /  chromosome, telomeric region / chromosome, telomeric region /  nuclear body / Ub-specific processing proteases / nuclear body / Ub-specific processing proteases /  cell division / cell division /  Golgi membrane / Golgi membrane /  Golgi apparatus / positive regulation of transcription by RNA polymerase II / zinc ion binding / Golgi apparatus / positive regulation of transcription by RNA polymerase II / zinc ion binding /  nucleoplasm / nucleoplasm /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.2 Å MOLECULAR REPLACEMENT / Resolution: 1.2 Å | ||||||
 Authors Authors | Musil, D. / Lehmann, D. / Buchstaller, H.-P. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2019 Journal: J.Med.Chem. / Year: 2019Title: Discovery and Optimization of 2-Arylquinazolin-4-ones into a Potent and Selective Tankyrase Inhibitor Modulating Wnt Pathway Activity. Authors: Buchstaller, H.P. / Anlauf, U. / Dorsch, D. / Kuhn, D. / Lehmann, M. / Leuthner, B. / Musil, D. / Radtki, D. / Ritzert, C. / Rohdich, F. / Schneider, R. / Esdar, C. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6qxu.cif.gz 6qxu.cif.gz | 106.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6qxu.ent.gz pdb6qxu.ent.gz | 84.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6qxu.json.gz 6qxu.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qx/6qxu https://data.pdbj.org/pub/pdb/validation_reports/qx/6qxu ftp://data.pdbj.org/pub/pdb/validation_reports/qx/6qxu ftp://data.pdbj.org/pub/pdb/validation_reports/qx/6qxu | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 24212.451 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: TNKS, PARP5A, PARPL, TIN1, TINF1, TNKS1 / Production host: Homo sapiens (human) / Gene: TNKS, PARP5A, PARPL, TIN1, TINF1, TNKS1 / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli)References: UniProt: O95271,  NAD+ ADP-ribosyltransferase, NAD+ ADP-ribosyltransferase,  Transferases; Glycosyltransferases; Pentosyltransferases Transferases; Glycosyltransferases; Pentosyltransferases |
|---|---|
| #2: Chemical | ChemComp-BME /  2-Mercaptoethanol 2-Mercaptoethanol |
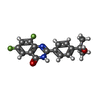
| #3: Chemical | ChemComp-JKN / |
| #4: Chemical | ChemComp-ZN / |
| #5: Water | ChemComp-HOH /  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.37 Å3/Da / Density % sol: 48.19 % |
|---|---|
Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 7 Details: 1.0 M Ammonium Sulfate, 0.1 M HEPES pH 7.0, 0.5% w/v PEG 8000 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X06SA / Wavelength: 0.999881 Å / Beamline: X06SA / Wavelength: 0.999881 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Feb 2, 2019 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.999881 Å / Relative weight: 1 : 0.999881 Å / Relative weight: 1 |
| Reflection | Resolution: 1.2→43.29 Å / Num. all: 438187 / Num. obs: 70543 / % possible obs: 96.6 % / Redundancy: 6.2 % / Biso Wilson estimate: 16.85 Å2 / CC1/2: 0.999 / Rrim(I) all: 0.053 / Net I/σ(I): 14.8 |
| Reflection shell | Resolution: 1.2→1.27 Å / Redundancy: 5.1 % / Mean I/σ(I) obs: 1.2 / Num. unique all: 56203 / Num. unique obs: 11113 / CC1/2: 0.713 / Rrim(I) all: 1.255 / % possible all: 95 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: In-house structure Resolution: 1.2→13.79 Å / Cor.coef. Fo:Fc: 0.97 / Cor.coef. Fo:Fc free: 0.964 / SU R Cruickshank DPI: 0.043 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.047 / SU Rfree Blow DPI: 0.049 / SU Rfree Cruickshank DPI: 0.045
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 22.59 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 1.2→13.79 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.2→1.21 Å / Total num. of bins used: 50
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: -21.2017 Å / Origin y: -18.6126 Å / Origin z: 4.035 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: { A|* } |
 Movie
Movie Controller
Controller












 PDBj
PDBj