+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5zhx | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of SmgGDS-558 and farnesylated RhoA complex | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  ONCOPROTEIN / armadillo GEF chaperone ONCOPROTEIN / armadillo GEF chaperone | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of ERK5 cascade / CAAX-box protein maturation / angiotensin-activated signaling pathway involved in heart process / RHOT1 GTPase cycle / RHOT2 GTPase cycle / regulation of matrix metallopeptidase secretion / vascular associated smooth muscle contraction / myosin filament assembly / positive regulation of mitochondrial calcium ion concentration / negative regulation of endoplasmic reticulum calcium ion concentration ...regulation of ERK5 cascade / CAAX-box protein maturation / angiotensin-activated signaling pathway involved in heart process / RHOT1 GTPase cycle / RHOT2 GTPase cycle / regulation of matrix metallopeptidase secretion / vascular associated smooth muscle contraction / myosin filament assembly / positive regulation of mitochondrial calcium ion concentration / negative regulation of endoplasmic reticulum calcium ion concentration / aortic valve formation / alpha-beta T cell lineage commitment / mitotic cleavage furrow formation / bone trabecula morphogenesis / positive regulation of lipase activity / endothelial tube lumen extension / skeletal muscle satellite cell migration / positive regulation of vascular associated smooth muscle contraction / angiotensin-mediated vasoconstriction involved in regulation of systemic arterial blood pressure / SLIT2:ROBO1 increases RHOA activity / RHO GTPases Activate Rhotekin and Rhophilins / Roundabout signaling pathway / negative regulation of intracellular steroid hormone receptor signaling pathway / Axonal growth inhibition (RHOA activation) / Axonal growth stimulation / regulation of neural precursor cell proliferation / cleavage furrow formation / regulation of modification of postsynaptic actin cytoskeleton / regulation of osteoblast proliferation / forebrain radial glial cell differentiation /  cell junction assembly / apical junction assembly / regulation of systemic arterial blood pressure by endothelin / cellular response to chemokine / negative regulation of cell migration involved in sprouting angiogenesis / beta selection / establishment of epithelial cell apical/basal polarity / regulation of modification of postsynaptic structure / negative regulation of cell size / RHO GTPases Activate ROCKs / negative regulation of oxidative phosphorylation / negative regulation of motor neuron apoptotic process / RHO GTPases activate CIT / cardiac muscle hypertrophy / PCP/CE pathway / Sema4D induced cell migration and growth-cone collapse / RHO GTPases activate KTN1 / apolipoprotein A-I-mediated signaling pathway / positive regulation of podosome assembly / regulation of mitochondrion organization / negative regulation of cell-substrate adhesion / cell junction assembly / apical junction assembly / regulation of systemic arterial blood pressure by endothelin / cellular response to chemokine / negative regulation of cell migration involved in sprouting angiogenesis / beta selection / establishment of epithelial cell apical/basal polarity / regulation of modification of postsynaptic structure / negative regulation of cell size / RHO GTPases Activate ROCKs / negative regulation of oxidative phosphorylation / negative regulation of motor neuron apoptotic process / RHO GTPases activate CIT / cardiac muscle hypertrophy / PCP/CE pathway / Sema4D induced cell migration and growth-cone collapse / RHO GTPases activate KTN1 / apolipoprotein A-I-mediated signaling pathway / positive regulation of podosome assembly / regulation of mitochondrion organization / negative regulation of cell-substrate adhesion /  Wnt signaling pathway, planar cell polarity pathway / Sema4D mediated inhibition of cell attachment and migration / positive regulation of alpha-beta T cell differentiation / ossification involved in bone maturation / Wnt signaling pathway, planar cell polarity pathway / Sema4D mediated inhibition of cell attachment and migration / positive regulation of alpha-beta T cell differentiation / ossification involved in bone maturation /  odontogenesis / motor neuron apoptotic process / odontogenesis / motor neuron apoptotic process /  wound healing, spreading of cells / negative regulation of GTPase activity / PI3K/AKT activation / positive regulation of leukocyte adhesion to vascular endothelial cell / apical junction complex / wound healing, spreading of cells / negative regulation of GTPase activity / PI3K/AKT activation / positive regulation of leukocyte adhesion to vascular endothelial cell / apical junction complex /  regulation of focal adhesion assembly / negative chemotaxis / regulation of focal adhesion assembly / negative chemotaxis /  myosin binding / EPHA-mediated growth cone collapse / myosin binding / EPHA-mediated growth cone collapse /  stress fiber assembly / regulation of neuron projection development / RHOC GTPase cycle / androgen receptor signaling pathway / positive regulation of cytokinesis / cellular response to cytokine stimulus / cerebral cortex cell migration / ERBB2 Regulates Cell Motility / stress fiber assembly / regulation of neuron projection development / RHOC GTPase cycle / androgen receptor signaling pathway / positive regulation of cytokinesis / cellular response to cytokine stimulus / cerebral cortex cell migration / ERBB2 Regulates Cell Motility /  cleavage furrow / semaphorin-plexin signaling pathway / Rho protein signal transduction / ficolin-1-rich granule membrane / protein localization to nucleus / cleavage furrow / semaphorin-plexin signaling pathway / Rho protein signal transduction / ficolin-1-rich granule membrane / protein localization to nucleus /  mitotic spindle assembly / RHOA GTPase cycle / endothelial cell migration / positive regulation of T cell migration / PTK6 Regulates RHO GTPases, RAS GTPase and MAP kinases / cytoplasmic microtubule organization / regulation of microtubule cytoskeleton organization / skeletal muscle tissue development / negative regulation of reactive oxygen species biosynthetic process / mitotic spindle assembly / RHOA GTPase cycle / endothelial cell migration / positive regulation of T cell migration / PTK6 Regulates RHO GTPases, RAS GTPase and MAP kinases / cytoplasmic microtubule organization / regulation of microtubule cytoskeleton organization / skeletal muscle tissue development / negative regulation of reactive oxygen species biosynthetic process /  regulation of cell migration / RHO GTPases activate PKNs / positive regulation of stress fiber assembly / GPVI-mediated activation cascade / EPHB-mediated forward signaling / substantia nigra development / positive regulation of neuron differentiation / cell-matrix adhesion / substrate adhesion-dependent cell spreading / guanyl-nucleotide exchange factor activity / regulation of cell migration / RHO GTPases activate PKNs / positive regulation of stress fiber assembly / GPVI-mediated activation cascade / EPHB-mediated forward signaling / substantia nigra development / positive regulation of neuron differentiation / cell-matrix adhesion / substrate adhesion-dependent cell spreading / guanyl-nucleotide exchange factor activity /  small monomeric GTPase / G protein activity small monomeric GTPase / G protein activitySimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 3.5 Å SYNCHROTRON / Resolution: 3.5 Å | ||||||
 Authors Authors | Shimizu, H. / Toma-Fukai, S. / Shimizu, T. | ||||||
 Citation Citation |  Journal: Proc. Natl. Acad. Sci. U.S.A. / Year: 2018 Journal: Proc. Natl. Acad. Sci. U.S.A. / Year: 2018Title: GEF mechanism revealed by the structure of SmgGDS-558 and farnesylated RhoA complex and its implication for a chaperone mechanism. Authors: Shimizu, H. / Toma-Fukai, S. / Kontani, K. / Katada, T. / Shimizu, T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5zhx.cif.gz 5zhx.cif.gz | 469.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5zhx.ent.gz pdb5zhx.ent.gz | 391.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5zhx.json.gz 5zhx.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zh/5zhx https://data.pdbj.org/pub/pdb/validation_reports/zh/5zhx ftp://data.pdbj.org/pub/pdb/validation_reports/zh/5zhx ftp://data.pdbj.org/pub/pdb/validation_reports/zh/5zhx | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments:
NCS ensembles :
|
- Components
Components
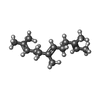
| #1: Protein | Mass: 53418.945 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: RAP1GDS1 / Production host: Homo sapiens (human) / Gene: RAP1GDS1 / Production host:   Escherichia coli (E. coli) / References: UniProt: P52306 Escherichia coli (E. coli) / References: UniProt: P52306#2: Protein |  / Rho cDNA clone 12 / h12 / Rho cDNA clone 12 / h12Mass: 22168.531 Da / Num. of mol.: 4 / Mutation: L193A Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: RHOA, ARH12, ARHA, RHO12 / Production host: Homo sapiens (human) / Gene: RHOA, ARH12, ARHA, RHO12 / Production host:   Escherichia coli (E. coli) / References: UniProt: P61586 Escherichia coli (E. coli) / References: UniProt: P61586#3: Chemical | ChemComp-FAR /  Farnesol Farnesol |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.99 Å3/Da / Density % sol: 58.89 % |
|---|---|
Crystal grow | Temperature: 283 K / Method: vapor diffusion, sitting drop / Details: 0.2M Sodium malonate, 20% PEG 3350 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SPring-8 SPring-8  / Beamline: BL44XU / Wavelength: 0.9 Å / Beamline: BL44XU / Wavelength: 0.9 Å |
| Detector | Type: RAYONIX MX300HE / Detector: CCD / Date: Oct 10, 2016 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.9 Å / Relative weight: 1 : 0.9 Å / Relative weight: 1 |
| Reflection | Resolution: 3.5→136.1 Å / Num. obs: 44892 / % possible obs: 99.9 % / Redundancy: 43.8 % / Biso Wilson estimate: 132.227 Å2 / Net I/σ(I): 10.4 |
| Reflection shell | Resolution: 3.5→3.59 Å |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 3.5→136.1 Å / Cor.coef. Fo:Fc: 0.93 / Cor.coef. Fo:Fc free: 0.9 / Cross valid method: THROUGHOUT / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 3.5→136.1 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj