+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4xg7 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of an inhibitor-bound Syk | ||||||
 Components Components | Tyrosine-protein kinase SYK | ||||||
 Keywords Keywords | TRANSFERASE/TRANSFERASE INHIBITOR / Syk kinase / Inhibitor /  Spleen tyrosine kinase / TRANSFERASE-TRANSFERASE INHIBITOR complex Spleen tyrosine kinase / TRANSFERASE-TRANSFERASE INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationserotonin secretion by platelet /  interleukin-15 receptor binding / positive regulation of interleukin-3 production / regulation of superoxide anion generation / regulation of neutrophil degranulation / regulation of arachidonic acid secretion / cellular response to lectin / gamma-delta T cell differentiation / positive regulation of gamma-delta T cell differentiation / interleukin-15 receptor binding / positive regulation of interleukin-3 production / regulation of superoxide anion generation / regulation of neutrophil degranulation / regulation of arachidonic acid secretion / cellular response to lectin / gamma-delta T cell differentiation / positive regulation of gamma-delta T cell differentiation /  B cell receptor complex ...serotonin secretion by platelet / B cell receptor complex ...serotonin secretion by platelet /  interleukin-15 receptor binding / positive regulation of interleukin-3 production / regulation of superoxide anion generation / regulation of neutrophil degranulation / regulation of arachidonic acid secretion / cellular response to lectin / gamma-delta T cell differentiation / positive regulation of gamma-delta T cell differentiation / interleukin-15 receptor binding / positive regulation of interleukin-3 production / regulation of superoxide anion generation / regulation of neutrophil degranulation / regulation of arachidonic acid secretion / cellular response to lectin / gamma-delta T cell differentiation / positive regulation of gamma-delta T cell differentiation /  B cell receptor complex / neutrophil activation involved in immune response / B cell receptor complex / neutrophil activation involved in immune response /  Toll-like receptor binding / Toll-like receptor binding /  regulation of platelet aggregation / positive regulation of alpha-beta T cell proliferation / leukocyte activation involved in immune response / positive regulation of mast cell cytokine production / regulation of platelet aggregation / positive regulation of alpha-beta T cell proliferation / leukocyte activation involved in immune response / positive regulation of mast cell cytokine production /  regulation of platelet activation / lymph vessel development / collagen-activated tyrosine kinase receptor signaling pathway / cell activation / positive regulation of mast cell degranulation / macrophage activation involved in immune response / beta selection / regulation of platelet activation / lymph vessel development / collagen-activated tyrosine kinase receptor signaling pathway / cell activation / positive regulation of mast cell degranulation / macrophage activation involved in immune response / beta selection /  regulation of phagocytosis / positive regulation of killing of cells of another organism / cellular response to molecule of fungal origin / regulation of tumor necrosis factor-mediated signaling pathway / leukotriene biosynthetic process / FLT3 signaling through SRC family kinases / early phagosome / positive regulation of monocyte chemotactic protein-1 production / interleukin-3-mediated signaling pathway / cellular response to lipid / positive regulation of granulocyte macrophage colony-stimulating factor production / positive regulation of cell adhesion mediated by integrin / regulation of DNA-binding transcription factor activity / Fc epsilon receptor (FCERI) signaling / Interleukin-2 signaling / positive regulation of alpha-beta T cell differentiation / blood vessel morphogenesis / regulation of phagocytosis / positive regulation of killing of cells of another organism / cellular response to molecule of fungal origin / regulation of tumor necrosis factor-mediated signaling pathway / leukotriene biosynthetic process / FLT3 signaling through SRC family kinases / early phagosome / positive regulation of monocyte chemotactic protein-1 production / interleukin-3-mediated signaling pathway / cellular response to lipid / positive regulation of granulocyte macrophage colony-stimulating factor production / positive regulation of cell adhesion mediated by integrin / regulation of DNA-binding transcription factor activity / Fc epsilon receptor (FCERI) signaling / Interleukin-2 signaling / positive regulation of alpha-beta T cell differentiation / blood vessel morphogenesis /  T cell receptor complex / positive regulation of B cell differentiation / leukocyte cell-cell adhesion / mast cell degranulation / Fc-gamma receptor signaling pathway involved in phagocytosis / Dectin-2 family / Fc-epsilon receptor signaling pathway / stimulatory C-type lectin receptor signaling pathway / T cell receptor complex / positive regulation of B cell differentiation / leukocyte cell-cell adhesion / mast cell degranulation / Fc-gamma receptor signaling pathway involved in phagocytosis / Dectin-2 family / Fc-epsilon receptor signaling pathway / stimulatory C-type lectin receptor signaling pathway /  phospholipase binding / positive regulation of cysteine-type endopeptidase activity involved in apoptotic process / positive regulation of interleukin-10 production / positive regulation of receptor internalization / cellular response to low-density lipoprotein particle stimulus / FCGR activation / positive regulation of type I interferon production / positive regulation of interleukin-4 production / positive regulation of bone resorption / phospholipase binding / positive regulation of cysteine-type endopeptidase activity involved in apoptotic process / positive regulation of interleukin-10 production / positive regulation of receptor internalization / cellular response to low-density lipoprotein particle stimulus / FCGR activation / positive regulation of type I interferon production / positive regulation of interleukin-4 production / positive regulation of bone resorption /  phosphatase binding / Role of phospholipids in phagocytosis / Role of LAT2/NTAL/LAB on calcium mobilization / positive regulation of calcium-mediated signaling / Signaling by CSF3 (G-CSF) / negative regulation of inflammatory response to antigenic stimulus / GPVI-mediated activation cascade / positive regulation of TORC1 signaling / phosphatase binding / Role of phospholipids in phagocytosis / Role of LAT2/NTAL/LAB on calcium mobilization / positive regulation of calcium-mediated signaling / Signaling by CSF3 (G-CSF) / negative regulation of inflammatory response to antigenic stimulus / GPVI-mediated activation cascade / positive regulation of TORC1 signaling /  extrinsic component of cytoplasmic side of plasma membrane / positive regulation of interleukin-12 production / extrinsic component of cytoplasmic side of plasma membrane / positive regulation of interleukin-12 production /  SH2 domain binding / phosphotyrosine residue binding / Integrin signaling / FCERI mediated Ca+2 mobilization / FCGR3A-mediated IL10 synthesis / regulation of ERK1 and ERK2 cascade / B cell differentiation / SH2 domain binding / phosphotyrosine residue binding / Integrin signaling / FCERI mediated Ca+2 mobilization / FCGR3A-mediated IL10 synthesis / regulation of ERK1 and ERK2 cascade / B cell differentiation /  neutrophil chemotaxis / positive regulation of superoxide anion generation / Antigen activates B Cell Receptor (BCR) leading to generation of second messengers / integrin-mediated signaling pathway / positive regulation of interleukin-8 production / calcium-mediated signaling / Regulation of signaling by CBL / positive regulation of protein-containing complex assembly / FCGR3A-mediated phagocytosis / FCERI mediated MAPK activation / animal organ morphogenesis / B cell receptor signaling pathway / neutrophil chemotaxis / positive regulation of superoxide anion generation / Antigen activates B Cell Receptor (BCR) leading to generation of second messengers / integrin-mediated signaling pathway / positive regulation of interleukin-8 production / calcium-mediated signaling / Regulation of signaling by CBL / positive regulation of protein-containing complex assembly / FCGR3A-mediated phagocytosis / FCERI mediated MAPK activation / animal organ morphogenesis / B cell receptor signaling pathway /  non-specific protein-tyrosine kinase / non-membrane spanning protein tyrosine kinase activity / Inactivation of CSF3 (G-CSF) signaling / non-specific protein-tyrosine kinase / non-membrane spanning protein tyrosine kinase activity / Inactivation of CSF3 (G-CSF) signaling /  receptor internalization / Regulation of actin dynamics for phagocytic cup formation / CLEC7A (Dectin-1) signaling / receptor internalization / Regulation of actin dynamics for phagocytic cup formation / CLEC7A (Dectin-1) signaling /  platelet activation / positive regulation of interleukin-6 production / protein import into nucleus / peptidyl-tyrosine phosphorylation / platelet activation / positive regulation of interleukin-6 production / protein import into nucleus / peptidyl-tyrosine phosphorylation /  cell surface receptor protein tyrosine kinase signaling pathway / positive regulation of peptidyl-tyrosine phosphorylation / cell surface receptor protein tyrosine kinase signaling pathway / positive regulation of peptidyl-tyrosine phosphorylation /  integrin binding / positive regulation of tumor necrosis factor production integrin binding / positive regulation of tumor necrosis factor productionSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.76 Å MOLECULAR REPLACEMENT / Resolution: 1.76 Å | ||||||
 Authors Authors | Lee, S.J. / Choi, J. / Han, B.G. / Song, H. / Koh, J.S. / Lee, B.I. | ||||||
 Citation Citation |  Journal: Febs J. / Year: 2016 Journal: Febs J. / Year: 2016Title: Crystal structures of spleen tyrosine kinase in complex with novel inhibitors: structural insights for design of anticancer drugs Authors: Lee, S.J. / Choi, J.S. / Han, B.G. / Kim, H.S. / Song, H.J. / Lee, J. / Nam, S. / Goh, S.H. / Kim, J.H. / Koh, J.S. / Lee, B.I. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4xg7.cif.gz 4xg7.cif.gz | 74.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4xg7.ent.gz pdb4xg7.ent.gz | 53.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4xg7.json.gz 4xg7.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/xg/4xg7 https://data.pdbj.org/pub/pdb/validation_reports/xg/4xg7 ftp://data.pdbj.org/pub/pdb/validation_reports/xg/4xg7 ftp://data.pdbj.org/pub/pdb/validation_reports/xg/4xg7 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4xg2SC  4xg3C  4xg4C  4xg6C  4xg8C  4xg9C  5ghvC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein |  / Spleen tyrosine kinase / p72-Syk / Spleen tyrosine kinase / p72-SykMass: 33900.965 Da / Num. of mol.: 1 / Fragment: protein kinase domain, residues 356-635 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: SYK / Plasmid: pVL1393 / Production host: Homo sapiens (human) / Gene: SYK / Plasmid: pVL1393 / Production host:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm)References: UniProt: P43405,  non-specific protein-tyrosine kinase non-specific protein-tyrosine kinase |
|---|---|
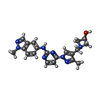
| #2: Chemical | ChemComp-X7G / |
| #3: Water | ChemComp-HOH /  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.13 Å3/Da / Density % sol: 42.16 % |
|---|---|
Crystal grow | Temperature: 278 K / Method: vapor diffusion, hanging drop / pH: 8.5 / Details: 10~20%(v/v) PEG3350, 100 mM Tris-HCl |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SPring-8 SPring-8  / Beamline: BL44XU / Wavelength: 0.9 Å / Beamline: BL44XU / Wavelength: 0.9 Å |
| Detector | Type: RAYONIX MX300HE / Detector: CCD / Date: Jun 29, 2011 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.9 Å / Relative weight: 1 : 0.9 Å / Relative weight: 1 |
| Reflection | Resolution: 1.76→50 Å / Num. obs: 27304 / % possible obs: 97.4 % / Redundancy: 6.2 % / Net I/σ(I): 58.15 |
| Reflection shell | Resolution: 1.76→1.79 Å |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4XG2 Resolution: 1.76→44.09 Å / Cor.coef. Fo:Fc: 0.949 / Cor.coef. Fo:Fc free: 0.914 / SU B: 5.791 / SU ML: 0.084 / Cross valid method: THROUGHOUT / ESU R: 0.265 / ESU R Free: 0.127 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 33.304 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.76→44.09 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller























 PDBj
PDBj