[English] 日本語
 Yorodumi
Yorodumi- PDB-4tu0: CRYSTAL STRUCTURE OF CHIKUNGUNYA VIRUS NSP3 MACRO DOMAIN IN COMPL... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4tu0 | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF CHIKUNGUNYA VIRUS NSP3 MACRO DOMAIN IN COMPLEX WITH A 2'-5' OLIGOADENYLATE TRIMER | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  VIRAL PROTEIN / VIRAL PROTEIN /  STRUCTURAL GENOMICS / MARSEILLES STRUCTURAL GENOMICS PROGRAM AT AFMB / MSGP / ATP-BINDING / STRUCTURAL GENOMICS / MARSEILLES STRUCTURAL GENOMICS PROGRAM AT AFMB / MSGP / ATP-BINDING /  CYTOPLASM / CYTOPLASM /  HELICASE / HELICASE /  HYDROLASE / HYDROLASE /  MEMBRANE / NUCLEOTIDE-BINDING / MEMBRANE / NUCLEOTIDE-BINDING /  NUCLEOTIDYLTRANSFERASE / NUCLEOTIDYLTRANSFERASE /  RNA REPLICATION / RNA-BINDING / RNA REPLICATION / RNA-BINDING /  RNA-DIRECTED RNA POLYMERASE RNA-DIRECTED RNA POLYMERASE | ||||||
| Function / homology |  Function and homology information Function and homology informationhost cell filopodium /  ADP-ribose 1''-phosphate phosphatase / mRNA methyltransferase activity / mRNA 5'-triphosphate monophosphatase activity / mRNA 5'-phosphatase / ADP-ribose 1''-phosphate phosphatase / mRNA methyltransferase activity / mRNA 5'-triphosphate monophosphatase activity / mRNA 5'-phosphatase /  polynucleotide 5'-phosphatase activity / polynucleotide 5'-phosphatase activity /  polynucleotide adenylyltransferase / poly(A) RNA polymerase activity / regulation of cytoskeleton organization / symbiont-mediated suppression of host mRNA transcription via inhibition of RNA polymerase II activity ...host cell filopodium / polynucleotide adenylyltransferase / poly(A) RNA polymerase activity / regulation of cytoskeleton organization / symbiont-mediated suppression of host mRNA transcription via inhibition of RNA polymerase II activity ...host cell filopodium /  ADP-ribose 1''-phosphate phosphatase / mRNA methyltransferase activity / mRNA 5'-triphosphate monophosphatase activity / mRNA 5'-phosphatase / ADP-ribose 1''-phosphate phosphatase / mRNA methyltransferase activity / mRNA 5'-triphosphate monophosphatase activity / mRNA 5'-phosphatase /  polynucleotide 5'-phosphatase activity / polynucleotide 5'-phosphatase activity /  polynucleotide adenylyltransferase / poly(A) RNA polymerase activity / regulation of cytoskeleton organization / symbiont-mediated suppression of host mRNA transcription via inhibition of RNA polymerase II activity / 7-methylguanosine mRNA capping / symbiont-mediated suppression of host JAK-STAT cascade via inhibition of STAT1 activity / cysteine-type peptidase activity / polynucleotide adenylyltransferase / poly(A) RNA polymerase activity / regulation of cytoskeleton organization / symbiont-mediated suppression of host mRNA transcription via inhibition of RNA polymerase II activity / 7-methylguanosine mRNA capping / symbiont-mediated suppression of host JAK-STAT cascade via inhibition of STAT1 activity / cysteine-type peptidase activity /  Transferases; Transferring one-carbon groups; Methyltransferases / host cell cytoplasmic vesicle membrane / nucleoside-triphosphate phosphatase / Transferases; Transferring one-carbon groups; Methyltransferases / host cell cytoplasmic vesicle membrane / nucleoside-triphosphate phosphatase /  Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases /  Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases / Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases /  RNA helicase activity / RNA helicase activity /  RNA helicase / RNA helicase /  RNA-directed RNA polymerase / viral RNA genome replication / RNA-directed RNA polymerase / viral RNA genome replication /  RNA-dependent RNA polymerase activity / DNA-templated transcription / symbiont-mediated suppression of host type I interferon-mediated signaling pathway / host cell nucleus / GTP binding / host cell plasma membrane / RNA-dependent RNA polymerase activity / DNA-templated transcription / symbiont-mediated suppression of host type I interferon-mediated signaling pathway / host cell nucleus / GTP binding / host cell plasma membrane /  ATP hydrolysis activity / ATP hydrolysis activity /  proteolysis / proteolysis /  RNA binding / RNA binding /  ATP binding / ATP binding /  membrane / membrane /  metal ion binding metal ion bindingSimilarity search - Function | ||||||
| Biological species |    Chikungunya virus Chikungunya virussynthetic construct (others) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.3 Å MOLECULAR REPLACEMENT / Resolution: 2.3 Å | ||||||
 Authors Authors | Morin, B. / Ferron, f.p. / Malet, h. / Coutard, b. / Canard, b. | ||||||
 Citation Citation |  Journal: TO BE PUBLISHED Journal: TO BE PUBLISHEDTitle: CRYSTAL STRUCTURE OF CHIKUNGUNYA VIRUS NSP3 MACRO DOMAIN IN COMPLEX WITH A 2'-5' OLIGOADENYLATE TRIMER Authors: Morin, B. / Ferron, F.P. / Malet, H. / Coutard, B. / Canard, B. | ||||||
| History |
|
- Structure visualization
Structure visualization
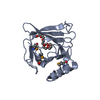
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4tu0.cif.gz 4tu0.cif.gz | 148 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4tu0.ent.gz pdb4tu0.ent.gz | 114 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4tu0.json.gz 4tu0.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/tu/4tu0 https://data.pdbj.org/pub/pdb/validation_reports/tu/4tu0 ftp://data.pdbj.org/pub/pdb/validation_reports/tu/4tu0 ftp://data.pdbj.org/pub/pdb/validation_reports/tu/4tu0 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3gpgS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| 4 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 18635.123 Da / Num. of mol.: 4 / Fragment: UNP RESIDUES 1334-1493 Source method: isolated from a genetically manipulated source Source: (gene. exp.)    Chikungunya virus / Strain: S27-African prototype / Plasmid: PDEST14 / Production host: Chikungunya virus / Strain: S27-African prototype / Plasmid: PDEST14 / Production host:   ESCHERICHIA COLI (E. coli) / Strain (production host): T7 EXPRESS IQ (BIOLABS-C3016) ESCHERICHIA COLI (E. coli) / Strain (production host): T7 EXPRESS IQ (BIOLABS-C3016)References: UniProt: Q8JUX6, Transferases; Transferring one-carbon groups; Methyltransferases, Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases, polynucleotide 5'- ...References: UniProt: Q8JUX6,  Transferases; Transferring one-carbon groups; Methyltransferases, Transferases; Transferring one-carbon groups; Methyltransferases,  Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases, Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases,  polynucleotide 5'-phosphatase, polynucleotide 5'-phosphatase,  Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases, nucleoside-triphosphate phosphatase, Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases, nucleoside-triphosphate phosphatase,  RNA helicase, RNA helicase,  RNA-directed RNA polymerase RNA-directed RNA polymerase#2: RNA chain | Mass: 1102.620 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) #3: Chemical |  Diethylene glycol Diethylene glycol#4: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.5 Å3/Da / Density % sol: 50.86 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion / pH: 7.4 Details: 46% PEG 600, 100MM HEPES, PH 7.4, VAPOR DIFFUSION, HANGING DROP, TEMPERATURE 293K PH range: 7.4 |
-Data collection
| Diffraction | Mean temperature: 293 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID23-1 / Wavelength: 0.86 Å / Beamline: ID23-1 / Wavelength: 0.86 Å |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Nov 13, 2008 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.86 Å / Relative weight: 1 : 0.86 Å / Relative weight: 1 |
| Reflection | Resolution: 2.3→75.67 Å / Num. obs: 32115 / % possible obs: 100 % / Observed criterion σ(I): 0 / Redundancy: 1 % / Net I/σ(I): 0 |
| Reflection shell | Resolution: 2.3→2.42 Å / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3GPG Resolution: 2.3→75.67 Å / Cor.coef. Fo:Fc: 0.952 / Cor.coef. Fo:Fc free: 0.915 / SU B: 6.272 / SU ML: 0.154 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.335 / ESU R Free: 0.231 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 29.58 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→75.67 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj



