[English] 日本語
 Yorodumi
Yorodumi- PDB-4rgr: Crystal Structure of Putative MarR Family Transcriptional Regulat... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4rgr | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of Putative MarR Family Transcriptional Regulator HcaR from Acinetobacter sp. ADP | ||||||
 Components Components | Repressor protein | ||||||
 Keywords Keywords |  TRANSCRIPTION / TRANSCRIPTION /  Structural Genomics / PSI-Biology / Midwest Center for Structural Genomics / MCSG / winged helix-turn-helix Structural Genomics / PSI-Biology / Midwest Center for Structural Genomics / MCSG / winged helix-turn-helix | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |   Acinetobacter sp. ADP1 (bacteria) Acinetobacter sp. ADP1 (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 2.302 Å SAD / Resolution: 2.302 Å | ||||||
 Authors Authors | Kim, Y. / Joachimiak, G. / Bigelow, L. / Cobb, G. / Joachimiak, A. / Midwest Center for Structural Genomics (MCSG) | ||||||
 Citation Citation |  Journal: To be Published / Year: 2014 Journal: To be Published / Year: 2014Title: Crystal Structure of Putative MarR Family Transcriptional Regulator HcaR from Acinetobacter sp. ADP Authors: Kim, Y. / Joachimiak, G. / Bigelow, L. / Cobb, G. / Joachimiak, A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4rgr.cif.gz 4rgr.cif.gz | 129.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4rgr.ent.gz pdb4rgr.ent.gz | 108.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4rgr.json.gz 4rgr.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/rg/4rgr https://data.pdbj.org/pub/pdb/validation_reports/rg/4rgr ftp://data.pdbj.org/pub/pdb/validation_reports/rg/4rgr ftp://data.pdbj.org/pub/pdb/validation_reports/rg/4rgr | HTTPS FTP |
|---|
-Related structure data
| Related structure data | |
|---|---|
| Similar structure data | |
| Other databases |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Details | to generate tetramer apply x,y,z and -x+1,-y,z to the asymmetric unit of dimer |
- Components
Components
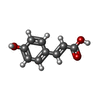
| #1: Protein |  / Repressor protein of the Hydroxycinnamate (Hca) catabolic genes / Repressor protein of the Hydroxycinnamate (Hca) catabolic genesMass: 18366.490 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Acinetobacter sp. ADP1 (bacteria) / Gene: ACIAD1728, hcaR / Plasmid: pMCSG19c / Production host: Acinetobacter sp. ADP1 (bacteria) / Gene: ACIAD1728, hcaR / Plasmid: pMCSG19c / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3) gold / References: UniProt: Q7X0D9 Escherichia coli (E. coli) / Strain (production host): BL21(DE3) gold / References: UniProt: Q7X0D9#2: Chemical |  P-Coumaric acid P-Coumaric acid#3: Chemical |  Sulfate Sulfate#4: Chemical | ChemComp-GOL / |  Glycerol Glycerol#5: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.3 Å3/Da / Density % sol: 46.47 % |
|---|---|
Crystal grow | Temperature: 289 K / Method: vapor diffusion, sitting drop / pH: 7 Details: 1.4 M Sodium Malonate pH 7.0, 0.1 M Bis-Tris Propane pH 7.0, 5 mM coumaric acid, VAPOR DIFFUSION, SITTING DROP, temperature 289K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 19-ID / Wavelength: 0.97935 Å / Beamline: 19-ID / Wavelength: 0.97935 Å |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Jun 18, 2009 / Details: mirrors |
| Radiation | Monochromator: double crystal monochromator / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.97935 Å / Relative weight: 1 : 0.97935 Å / Relative weight: 1 |
| Reflection | Resolution: 2.3→50 Å / Num. all: 15489 / Num. obs: 15489 / % possible obs: 99.7 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 7.9 % / Biso Wilson estimate: 44.06 Å2 / Rsym value: 0.09 / Net I/σ(I): 14.1 |
| Reflection shell | Resolution: 2.3→2.34 Å / Redundancy: 8.1 % / Mean I/σ(I) obs: 4.8 / Num. unique all: 747 / Rsym value: 0.499 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  SAD / Resolution: 2.302→39.576 Å / SU ML: 0.24 / Isotropic thermal model: mixed / Cross valid method: THROUGHOUT / σ(F): 0 / Phase error: 21 / Stereochemistry target values: MLHL SAD / Resolution: 2.302→39.576 Å / SU ML: 0.24 / Isotropic thermal model: mixed / Cross valid method: THROUGHOUT / σ(F): 0 / Phase error: 21 / Stereochemistry target values: MLHL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 49.5 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.302→39.576 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj