[English] 日本語
 Yorodumi
Yorodumi- PDB-4aq7: Ternary complex of E. coli leucyl-tRNA synthetase, tRNA(leu) and ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4aq7 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Ternary complex of E. coli leucyl-tRNA synthetase, tRNA(leu) and leucyl-adenylate analogue in the aminoacylation conformation | ||||||
 Components Components |
| ||||||
 Keywords Keywords | LIGASE/RNA / LIGASE-RNA COMPLEX /  LIGASE / NUCLEOTIDE(ATP)-BINDING / LIGASE / NUCLEOTIDE(ATP)-BINDING /  PROTEIN BIOSYNTHESIS / CLASS I AMINOACYL-TRNA SYNTHETASE / ATP-BINDING / METAL-BINDING PROTEIN BIOSYNTHESIS / CLASS I AMINOACYL-TRNA SYNTHETASE / ATP-BINDING / METAL-BINDING | ||||||
| Function / homology |  Function and homology information Function and homology information leucine-tRNA ligase / leucine-tRNA ligase /  leucine-tRNA ligase activity / leucyl-tRNA aminoacylation / aminoacyl-tRNA editing activity / leucine-tRNA ligase activity / leucyl-tRNA aminoacylation / aminoacyl-tRNA editing activity /  ATP binding / ATP binding /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   ESCHERICHIA COLI (E. coli) ESCHERICHIA COLI (E. coli) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.5 Å MOLECULAR REPLACEMENT / Resolution: 2.5 Å | ||||||
 Authors Authors | Palencia, A. / Crepin, T. / Vu, M.T. / Lincecum Jr, T.L. / Martinis, S.A. / Cusack, S. | ||||||
 Citation Citation |  Journal: Nat.Struct.Mol.Biol. / Year: 2012 Journal: Nat.Struct.Mol.Biol. / Year: 2012Title: Structural Dynamics of the Aminoacylation and Proofreading Functional Cycle of Bacterial Leucyl-tRNA Synthetase Authors: Palencia, A. / Crepin, T. / Vu, M.T. / Lincecum Jr, T.L. / Martinis, S.A. / Cusack, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4aq7.cif.gz 4aq7.cif.gz | 872.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4aq7.ent.gz pdb4aq7.ent.gz | 713.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4aq7.json.gz 4aq7.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/aq/4aq7 https://data.pdbj.org/pub/pdb/validation_reports/aq/4aq7 ftp://data.pdbj.org/pub/pdb/validation_reports/aq/4aq7 ftp://data.pdbj.org/pub/pdb/validation_reports/aq/4aq7 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4arcC  4ariC  4as1C  1h3nS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein / RNA chain , 2 types, 4 molecules ADBE
| #1: Protein | Mass: 99516.016 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   ESCHERICHIA COLI (E. coli) / Production host: ESCHERICHIA COLI (E. coli) / Production host:   ESCHERICHIA COLI (E. coli) / Strain (production host): BL21 / References: UniProt: P07813, ESCHERICHIA COLI (E. coli) / Strain (production host): BL21 / References: UniProt: P07813,  leucine-tRNA ligase leucine-tRNA ligase#2: RNA chain | Mass: 28052.652 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.)   ESCHERICHIA COLI (E. coli) ESCHERICHIA COLI (E. coli) |
|---|
-Non-polymers , 5 types, 177 molecules 
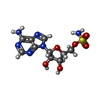







| #3: Chemical | | #4: Chemical | #5: Chemical |  Leucine Leucine#6: Chemical | #7: Water | ChemComp-HOH / |  Water Water |
|---|
-Details
| Compound details | ATP + L-LEUCINE + TRNA (GIVES AMP + PPI + L-LEUCYL-TRNA(LEU) |
|---|---|
| Nonpolymer details | [(2R,3S,4R,5R)-5-(6-AMINO-9H-PURIN-9-YL)-3, 4-DIHYDROXYTETRAHYDRO-2-FURANYL]METHYL SULFAMATE (LMS): ...[(2R,3S,4R,5R)-5-(6-AMINO-9H-PURIN-9-YL)-3, 4-DIHYDROXYT |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 2 X-RAY DIFFRACTION / Number of used crystals: 2 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.9 Å3/Da / Density % sol: 42 % / Description: NONE |
|---|---|
Crystal grow | pH: 5.5 Details: 33 MICROM LEURS, 50 MICROM TRNALEU AND 1 MM LEUCYL-ADENYLATE ANALOGUE MIXED EQUALLY WITH 0.1 M BIS-TRIS PH 5.5, 23-25% W/V PEG 3350, AND 200 MM AMMONIUM ACETATE |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID14-4 / Wavelength: 0.9375 / Beamline: ID14-4 / Wavelength: 0.9375 |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Nov 29, 2009 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.9375 Å / Relative weight: 1 : 0.9375 Å / Relative weight: 1 |
| Reflection | Resolution: 2.5→50 Å / Num. obs: 75711 / % possible obs: 90.3 % / Observed criterion σ(I): 0 / Redundancy: 2.9 % / Rmerge(I) obs: 0.08 / Net I/σ(I): 11.1 |
| Reflection shell | Resolution: 2.5→2.6 Å / Redundancy: 2.16 % / Rmerge(I) obs: 0.52 / Mean I/σ(I) obs: 2.1 / % possible all: 76.6 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1H3N Resolution: 2.5→39.69 Å / Cor.coef. Fo:Fc: 0.951 / Cor.coef. Fo:Fc free: 0.913 / SU B: 23.548 / SU ML: 0.244 / Cross valid method: THROUGHOUT / ESU R: 1.175 / ESU R Free: 0.322 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. U VALUES WITH TLS ADDED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 48.175 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.5→39.69 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller















 PDBj
PDBj



































