[English] 日本語
 Yorodumi
Yorodumi- PDB-2vt4: TURKEY BETA1 ADRENERGIC RECEPTOR WITH STABILISING MUTATIONS AND B... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2vt4 | ||||||
|---|---|---|---|---|---|---|---|
| Title | TURKEY BETA1 ADRENERGIC RECEPTOR WITH STABILISING MUTATIONS AND BOUND CYANOPINDOLOL | ||||||
 Components Components | BETA1 ADRENERGIC RECEPTOR | ||||||
 Keywords Keywords |  RECEPTOR / RECEPTOR /  GPCR / GPCR /  MEMBRANE / MEMBRANE /  PALMITATE / PALMITATE /  TRANSDUCER / ANTAGONIST BOUND FORM / INTEGRAL MEMBRANE PROTEIN / TRANSDUCER / ANTAGONIST BOUND FORM / INTEGRAL MEMBRANE PROTEIN /  G-PROTEIN COUPLED RECEPTOR / G-PROTEIN COUPLED RECEPTOR /  G PROTEIN COUPLED RECEPTOR / THERMOSTABILISING POINT MUTATIONS / G PROTEIN COUPLED RECEPTOR / THERMOSTABILISING POINT MUTATIONS /  PHOSPHOPROTEIN / SEVEN-HELIX RECEPTOR / PHOSPHOPROTEIN / SEVEN-HELIX RECEPTOR /  LIPOPROTEIN / LIPOPROTEIN /  7TM RECEPTOR / 7TM RECEPTOR /  GLYCOPROTEIN / GLYCOPROTEIN /  TRANSMEMBRANE TRANSMEMBRANE | ||||||
| Function / homology |  Function and homology information Function and homology informationbeta1-adrenergic receptor activity / positive regulation of heart contraction / regulation of circadian sleep/wake cycle, sleep / adenylate cyclase-activating adrenergic receptor signaling pathway / positive regulation of GTPase activity /  early endosome / early endosome /  membrane / identical protein binding / membrane / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | ||||||
| Biological species |   MELEAGRIS GALLOPAVO (turkey) MELEAGRIS GALLOPAVO (turkey) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.7 Å MOLECULAR REPLACEMENT / Resolution: 2.7 Å | ||||||
 Authors Authors | Warne, A. / Serrano-Vega, M.J. / Baker, J.G. / Moukhametzianov, R. / Edwards, P.C. / Henderson, R. / Leslie, A.G.W. / Tate, C.G. / Schertler, G.F.X. | ||||||
 Citation Citation |  Journal: Nature / Year: 2008 Journal: Nature / Year: 2008Title: Structure of a Beta1-Adrenergic G-Protein-Coupled Receptor. Authors: Warne, A. / Serrano-Vega, M.J. / Baker, J.G. / Moukhametzianov, R. / Edwards, P.C. / Henderson, R. / Leslie, A.G.W. / Tate, C.G. / Schertler, G.F.X. #1: Journal: Proc.Natl.Acad.Sci.USA / Year: 2008 Title: Conformational Thermostabilisation of the Beta1-Adrenergic Receptor in a Detergent Resistant Form Authors: Serrano-Vega, M.J. / Magnani, F. / Shibata, Y. / Tate, C.G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2vt4.cif.gz 2vt4.cif.gz | 233.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2vt4.ent.gz pdb2vt4.ent.gz | 188 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2vt4.json.gz 2vt4.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/vt/2vt4 https://data.pdbj.org/pub/pdb/validation_reports/vt/2vt4 ftp://data.pdbj.org/pub/pdb/validation_reports/vt/2vt4 ftp://data.pdbj.org/pub/pdb/validation_reports/vt/2vt4 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2rh1S S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| 4 | 
| ||||||||
| 5 | 
| ||||||||
| 6 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein / Sugars , 2 types, 16 molecules ABCD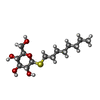

| #1: Protein | Mass: 35752.598 Da / Num. of mol.: 4 / Fragment: RESIDUES 33-243,272-276,279-367 / Mutation: YES Source method: isolated from a genetically manipulated source Source: (gene. exp.)   MELEAGRIS GALLOPAVO (turkey) / Cell: ERYTHROCYTE / Plasmid: PBACPAK8 / Cell line (production host): High Five / Production host: MELEAGRIS GALLOPAVO (turkey) / Cell: ERYTHROCYTE / Plasmid: PBACPAK8 / Cell line (production host): High Five / Production host:   TRICHOPLUSIA NI (cabbage looper) / References: UniProt: P07700 TRICHOPLUSIA NI (cabbage looper) / References: UniProt: P07700#4: Sugar | ChemComp-SOG /  N-Octyl beta-D-thioglucopyranoside N-Octyl beta-D-thioglucopyranoside |
|---|
-Non-polymers , 4 types, 41 molecules 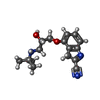






| #2: Chemical | ChemComp-P32 / #3: Chemical | ChemComp-NA / #5: Chemical |  Decane Decane#6: Water | ChemComp-HOH / |  Water Water |
|---|
-Details
| Compound details | ENGINEERED RESIDUE IN CHAIN A, ARG 68 TO SER ENGINEERED RESIDUE IN CHAIN A, MET 90 TO VAL ...ENGINEERED |
|---|---|
| Sequence details | RESIDUES 3-32 AT THE N-TERMINUS AND RESIDUES 244-271 AND 277-278 OF THE THIRD INTRCELLULAR LOOP ...RESIDUES 3-32 AT THE N-TERMINUS AND RESIDUES 244-271 AND 277-278 OF THE THIRD INTRCELLUL |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.94 Å3/Da / Density % sol: 58 % Description: THE LYSOZYME COMPONENT OF 2RH1 WAS REMOVED FOR THE MOLECULAR REPLACEMENT |
|---|---|
Crystal grow | Method: vapor diffusion / pH: 7.1 Details: VAPOUR DIFFUSION. EQUAL VOLUMES OF PROTEIN (6MG/ML) IN 10MM TRIS-HCL PH7.7, 50MM NACL, 0.1MM EDTA, 0.35% OCTYLTHIOGLUCOSIDE, 0.5MM CYANOPINDOLOL AND RESERVOIR 0.1M ADA, PH 6.9-7.3, 29-32% PEG600. |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID23-2 / Wavelength: 0.8726 / Beamline: ID23-2 / Wavelength: 0.8726 |
| Detector | Type: MARRESEARCH / Detector: CCD / Date: Dec 14, 2007 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.8726 Å / Relative weight: 1 : 0.8726 Å / Relative weight: 1 |
| Reflection | Resolution: 2.7→46.4 Å / Num. obs: 41499 / % possible obs: 96.2 % / Observed criterion σ(I): 0 / Redundancy: 1.8 % / Biso Wilson estimate: 39.32 Å2 / Rmerge(I) obs: 0.14 / Net I/σ(I): 5.8 |
| Reflection shell | Resolution: 2.7→2.85 Å / Redundancy: 1.8 % / Rmerge(I) obs: 0.67 / Mean I/σ(I) obs: 1.5 / % possible all: 95.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 2RH1 Resolution: 2.7→45.1 Å / SU ML: 0.44 / Phase error: 25.37 / Stereochemistry target values: ML Details: STRICT NCS, SIGMA 0.025, APPLIED TO CHAINS A AND D AND TO CHAINS B AND C, EXCLUDING DETERGENTS
| ||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL / Bsol: 52.82 Å2 / ksol: 0.343 e/Å3 | ||||||||||||||||||||||||
| Displacement parameters | Biso mean: 46.32 Å2
| ||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.7→45.1 Å
| ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj











