[English] 日本語
 Yorodumi
Yorodumi- PDB-2ieh: Crystal structure of human kinesin Eg5 in complex with (R)-mon97,... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2ieh | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of human kinesin Eg5 in complex with (R)-mon97, a new monastrol-based inhibitor that binds as (R)-enantiomer | ||||||
 Components Components | Kinesin-like protein KIF11 | ||||||
 Keywords Keywords |  HYDROLASE / beta-sheet core / flanked by three alpha-helices on each side HYDROLASE / beta-sheet core / flanked by three alpha-helices on each side | ||||||
| Function / homology |  Function and homology information Function and homology informationspindle elongation / Kinesins / plus-end-directed microtubule motor activity / regulation of mitotic centrosome separation / mitotic centrosome separation / COPI-dependent Golgi-to-ER retrograde traffic /  kinesin complex / kinesin complex /  microtubule motor activity / spindle organization / microtubule-based movement ...spindle elongation / Kinesins / plus-end-directed microtubule motor activity / regulation of mitotic centrosome separation / mitotic centrosome separation / COPI-dependent Golgi-to-ER retrograde traffic / microtubule motor activity / spindle organization / microtubule-based movement ...spindle elongation / Kinesins / plus-end-directed microtubule motor activity / regulation of mitotic centrosome separation / mitotic centrosome separation / COPI-dependent Golgi-to-ER retrograde traffic /  kinesin complex / kinesin complex /  microtubule motor activity / spindle organization / microtubule-based movement / microtubule motor activity / spindle organization / microtubule-based movement /  mitotic spindle assembly / MHC class II antigen presentation / mitotic spindle organization / spindle / mitotic spindle assembly / MHC class II antigen presentation / mitotic spindle organization / spindle /  mitotic spindle / mitotic spindle /  spindle pole / mitotic cell cycle / spindle pole / mitotic cell cycle /  microtubule binding / microtubule binding /  microtubule / microtubule /  cell division / cell division /  protein kinase binding / protein-containing complex / protein kinase binding / protein-containing complex /  ATP binding / ATP binding /  membrane / membrane /  nucleus / nucleus /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.7 Å MOLECULAR REPLACEMENT / Resolution: 2.7 Å | ||||||
 Authors Authors | Garcia-Saez, I. / Kozielski, F. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2007 Journal: J.Biol.Chem. / Year: 2007Title: Structure of human Eg5 in complex with a new monastrol-based inhibitor bound in the R configuration. Authors: Garcia-Saez, I. / DeBonis, S. / Lopez, R. / Trucco, F. / Rousseau, B. / Thuery, P. / Kozielski, F. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2ieh.cif.gz 2ieh.cif.gz | 154.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2ieh.ent.gz pdb2ieh.ent.gz | 120.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2ieh.json.gz 2ieh.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ie/2ieh https://data.pdbj.org/pub/pdb/validation_reports/ie/2ieh ftp://data.pdbj.org/pub/pdb/validation_reports/ie/2ieh ftp://data.pdbj.org/pub/pdb/validation_reports/ie/2ieh | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1q0bS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein |  / Kinesin-related motor protein Eg5 / Kinesin-like spindle protein HKSP / Thyroid receptor- ...Kinesin-related motor protein Eg5 / Kinesin-like spindle protein HKSP / Thyroid receptor-interacting protein 5 / TRIP5 / Kinesin-like protein 1 / Kinesin-related motor protein Eg5 / Kinesin-like spindle protein HKSP / Thyroid receptor- ...Kinesin-related motor protein Eg5 / Kinesin-like spindle protein HKSP / Thyroid receptor-interacting protein 5 / TRIP5 / Kinesin-like protein 1Mass: 40924.387 Da / Num. of mol.: 2 / Fragment: motor domain of human kinesin Eg5 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: KIF11, EG5, KNSL1 / Plasmid: pET28a / Production host: Homo sapiens (human) / Gene: KIF11, EG5, KNSL1 / Plasmid: pET28a / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3)pLys / References: UniProt: P52732, EC: 3.6.4.4 Escherichia coli (E. coli) / Strain (production host): BL21(DE3)pLys / References: UniProt: P52732, EC: 3.6.4.4 |
|---|
-Non-polymers , 7 types, 223 molecules 



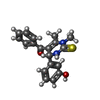








| #2: Chemical | | #3: Chemical | ChemComp-K / | #4: Chemical | ChemComp-CL / |  Chloride Chloride#5: Chemical |  Adenosine diphosphate Adenosine diphosphate#6: Chemical | #7: Chemical | ChemComp-PG4 / |  Polyethylene glycol Polyethylene glycol#8: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.7 Å3/Da / Density % sol: 54.39 % |
|---|---|
Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 5.6 Details: 20%PEG4000, 0.2M K2HPO4, 0.1M MES, pH 5.6, VAPOR DIFFUSION, HANGING DROP, temperature 277K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: BM30A / Wavelength: 0.979645 Å / Beamline: BM30A / Wavelength: 0.979645 Å |
| Detector | Type: CUSTOM-MADE / Detector: CCD / Date: Apr 10, 2006 / Details: mirrors |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.979645 Å / Relative weight: 1 : 0.979645 Å / Relative weight: 1 |
| Reflection | Resolution: 2.6→50 Å / Num. all: 52753 / Num. obs: 51576 / % possible obs: 97.8 % / Observed criterion σ(F): 2 / Observed criterion σ(I): 1 / Redundancy: 4 % / Rsym value: 0.19 / Net I/σ(I): 7.71 |
| Reflection shell | Resolution: 2.6→2.7 Å / Redundancy: 2.9 % / Mean I/σ(I) obs: 2.95 / Num. unique all: 2692 / Rsym value: 0.79 / % possible all: 84.3 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1Q0B Resolution: 2.7→25 Å / Cross valid method: THROUGHOUT / σ(F): 2 / Stereochemistry target values: Engh & Huber
| |||||||||||||||||||||||||
| Refine analyze |
| |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.7→25 Å
| |||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.7→2.87 Å / Rfactor Rfree error: 0.017
|
 Movie
Movie Controller
Controller












 PDBj
PDBj


















