+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2f4v | ||||||
|---|---|---|---|---|---|---|---|
| Title | 30S ribosome + designer antibiotic | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  RIBOSOME / 30S ribosome subunit / designer antibiotic RIBOSOME / 30S ribosome subunit / designer antibiotic | ||||||
| Function / homology |  Function and homology information Function and homology informationsmall ribosomal subunit /  tRNA binding / tRNA binding /  rRNA binding / rRNA binding /  ribosome / structural constituent of ribosome / ribosome / structural constituent of ribosome /  translation / translation /  ribonucleoprotein complex / response to antibiotic / ribonucleoprotein complex / response to antibiotic /  mRNA binding / zinc ion binding ...small ribosomal subunit / mRNA binding / zinc ion binding ...small ribosomal subunit /  tRNA binding / tRNA binding /  rRNA binding / rRNA binding /  ribosome / structural constituent of ribosome / ribosome / structural constituent of ribosome /  translation / translation /  ribonucleoprotein complex / response to antibiotic / ribonucleoprotein complex / response to antibiotic /  mRNA binding / zinc ion binding / mRNA binding / zinc ion binding /  metal ion binding / metal ion binding /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |    Thermus thermophilus (bacteria) Thermus thermophilus (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.8 Å MOLECULAR REPLACEMENT / Resolution: 3.8 Å | ||||||
 Authors Authors | Murray, J.B. / Meroueh, S.O. / Russell, R.J. / Lentzen, G. / Haddad, J. / Mobashery, S. | ||||||
 Citation Citation |  Journal: Chem.Biol. / Year: 2006 Journal: Chem.Biol. / Year: 2006Title: Interactions of designer antibiotics and the bacterial ribosome aminoacyl-tRNA site Authors: Murray, J.B. / Meroueh, S.O. / Russell, R.J. / Lentzen, G. / Haddad, J. / Mobashery, S. | ||||||
| History |
| ||||||
| Remark 999 | SEQUENCE THERE ARE SEQUENCE LINK PROBLEMS IN CHAIN A. SOME RESIDUES ARE NOT AT LINK DISTANCE, O3*-P ...SEQUENCE THERE ARE SEQUENCE LINK PROBLEMS IN CHAIN A. SOME RESIDUES ARE NOT AT LINK DISTANCE, O3*-P BOND DISTANCES ARE MORE THAN 3A LONG. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2f4v.cif.gz 2f4v.cif.gz | 1.3 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2f4v.ent.gz pdb2f4v.ent.gz | 1 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2f4v.json.gz 2f4v.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/f4/2f4v https://data.pdbj.org/pub/pdb/validation_reports/f4/2f4v ftp://data.pdbj.org/pub/pdb/validation_reports/f4/2f4v ftp://data.pdbj.org/pub/pdb/validation_reports/f4/2f4v | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-RNA chain , 2 types, 2 molecules AZ
| #1: RNA chain |  Mass: 490595.438 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: Thermus thermophilus (bacteria) / References:  GenBank: 155076 GenBank: 155076 |
|---|---|
| #2: RNA chain | Mass: 1178.722 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) Thermus thermophilus (bacteria) |
-30S ribosomal protein ... , 19 types, 19 molecules BCDEFGHIJKLMNOPQRST
| #3: Protein |  Mass: 29317.703 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P80371 Thermus thermophilus (bacteria) / References: UniProt: P80371 |
|---|---|
| #4: Protein |  Mass: 26751.076 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P80372 Thermus thermophilus (bacteria) / References: UniProt: P80372 |
| #5: Protein |  Mass: 24373.447 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P80373 Thermus thermophilus (bacteria) / References: UniProt: P80373 |
| #6: Protein |  Mass: 17583.416 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P27152, UniProt: Q5SHQ5*PLUS Thermus thermophilus (bacteria) / References: UniProt: P27152, UniProt: Q5SHQ5*PLUS |
| #7: Protein |  / TS9 / TS9Mass: 11988.753 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P23370, UniProt: Q5SLP8*PLUS Thermus thermophilus (bacteria) / References: UniProt: P23370, UniProt: Q5SLP8*PLUS |
| #8: Protein |  Mass: 18050.973 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P17291 Thermus thermophilus (bacteria) / References: UniProt: P17291 |
| #9: Protein |  Mass: 15868.570 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P24319, UniProt: P0DOY9*PLUS Thermus thermophilus (bacteria) / References: UniProt: P24319, UniProt: P0DOY9*PLUS |
| #10: Protein |  Mass: 14429.661 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P62669, UniProt: P80374*PLUS Thermus thermophilus (bacteria) / References: UniProt: P62669, UniProt: P80374*PLUS |
| #11: Protein |  Mass: 11954.968 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P80375, UniProt: Q5SHN7*PLUS Thermus thermophilus (bacteria) / References: UniProt: P80375, UniProt: Q5SHN7*PLUS |
| #12: Protein |  Mass: 13737.868 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P80376 Thermus thermophilus (bacteria) / References: UniProt: P80376 |
| #13: Protein |  Mass: 14637.384 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P17293, UniProt: Q5SHN3*PLUS Thermus thermophilus (bacteria) / References: UniProt: P17293, UniProt: Q5SHN3*PLUS |
| #14: Protein |  Mass: 14338.861 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P80377 Thermus thermophilus (bacteria) / References: UniProt: P80377 |
| #15: Protein |  Mass: 7158.725 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P24320, UniProt: P0DOY6*PLUS Thermus thermophilus (bacteria) / References: UniProt: P24320, UniProt: P0DOY6*PLUS |
| #16: Protein |  Mass: 10578.407 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P80378, UniProt: Q5SJ76*PLUS Thermus thermophilus (bacteria) / References: UniProt: P80378, UniProt: Q5SJ76*PLUS |
| #17: Protein |  Mass: 10409.983 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: Q5SJH3 Thermus thermophilus (bacteria) / References: UniProt: Q5SJH3 |
| #18: Protein |  Mass: 12324.670 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P24321, UniProt: P0DOY7*PLUS Thermus thermophilus (bacteria) / References: UniProt: P24321, UniProt: P0DOY7*PLUS |
| #19: Protein |  Mass: 10244.272 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P80382, UniProt: Q5SLQ0*PLUS Thermus thermophilus (bacteria) / References: UniProt: P80382, UniProt: Q5SLQ0*PLUS |
| #20: Protein |  Mass: 10605.464 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P80381, UniProt: Q5SHP2*PLUS Thermus thermophilus (bacteria) / References: UniProt: P80381, UniProt: Q5SHP2*PLUS |
| #21: Protein |  Mass: 11722.116 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)    Thermus thermophilus (bacteria) / References: UniProt: P62661, UniProt: P80380*PLUS Thermus thermophilus (bacteria) / References: UniProt: P62661, UniProt: P80380*PLUS |
-Non-polymers , 5 types, 117 molecules 

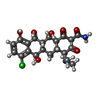
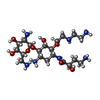





| #22: Chemical | ChemComp-MG / #23: Chemical | ChemComp-K / #24: Chemical | ChemComp-D2C / ( | #25: Chemical | ChemComp-AB9 / ( | #26: Chemical | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 4.62 Å3/Da / Density % sol: 73.36 % |
|---|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID29 / Wavelength: 0.991 Å / Beamline: ID29 / Wavelength: 0.991 Å |
| Detector | Date: Jun 1, 2002 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.991 Å / Relative weight: 1 : 0.991 Å / Relative weight: 1 |
| Reflection | Resolution: 3.8→30 Å / Num. all: 140000 / Num. obs: 138452 / % possible obs: 97.4 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Rmerge(I) obs: 0.161 |
| Reflection shell | Resolution: 3.8→3.93 Å / Rmerge(I) obs: 0.68 / % possible all: 96.2 |
- Processing
Processing
| Software | Name: REFMAC / Version: 5.1.24 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT / Resolution: 3.8→30 Å / Cor.coef. Fo:Fc: 0.82 / Cor.coef. Fo:Fc free: 0.781 / SU B: 39.167 / SU ML: 0.552 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R Free: 0.77 / Stereochemistry target values: MAXIMUM LIKELIHOOD MOLECULAR REPLACEMENT / Resolution: 3.8→30 Å / Cor.coef. Fo:Fc: 0.82 / Cor.coef. Fo:Fc free: 0.781 / SU B: 39.167 / SU ML: 0.552 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R Free: 0.77 / Stereochemistry target values: MAXIMUM LIKELIHOODDetails: There are sequence link problems in chain A. Some residues are not at link distance, O3*-P bond distances are more than 3A long.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: BABINET MODEL WITH MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 84.895 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.8→30 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 3.8→3.901 Å / Total num. of bins used: 20 /
|
 Movie
Movie Controller
Controller




















 PDBj
PDBj


































