[English] 日本語
 Yorodumi
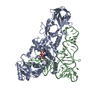
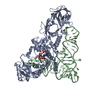
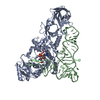
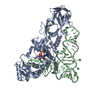
Yorodumi- PDB-1qrs: GLUTAMINYL-TRNA SYNTHETASE MUTANT D235N COMPLEXED WITH GLUTAMINE ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1qrs | ||||||
|---|---|---|---|---|---|---|---|
| Title | GLUTAMINYL-TRNA SYNTHETASE MUTANT D235N COMPLEXED WITH GLUTAMINE TRANSFER RNA | ||||||
 Components Components |
| ||||||
 Keywords Keywords | LIGASE/RNA / AMINOACYL-TRNA SYNTHASE /  PROTEIN BIOSYNTHESIS / PROTEIN BIOSYNTHESIS /  LIGASE / ATP-B / COMPLEX (AMINOACYL-TRNA SYNTHASE-TRNA) / LIGASE-RNA COMPLEX LIGASE / ATP-B / COMPLEX (AMINOACYL-TRNA SYNTHASE-TRNA) / LIGASE-RNA COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology information glutamine-tRNA ligase / glutamine-tRNA ligase /  glutamine-tRNA ligase activity / glutaminyl-tRNA aminoacylation / glutamyl-tRNA aminoacylation / glutamine-tRNA ligase activity / glutaminyl-tRNA aminoacylation / glutamyl-tRNA aminoacylation /  ATP binding / ATP binding /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   Escherichia coli (E. coli) Escherichia coli (E. coli) | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.6 Å X-RAY DIFFRACTION / Resolution: 2.6 Å | ||||||
 Authors Authors | Arnez, J.G. / Steitz, T.A. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 1996 Journal: Biochemistry / Year: 1996Title: Crystal structures of three misacylating mutants of Escherichia coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP. Authors: Arnez, J.G. / Steitz, T.A. #1:  Journal: Nature / Year: 1991 Journal: Nature / Year: 1991Title: Structural Basis of Anticodon Loop Recognition by Glutaminyl-tRNA Synthetase Authors: Rould, M.A. / Perona, J.J. / Steitz, T.A. #2:  Journal: Science / Year: 1989 Journal: Science / Year: 1989Title: Structural Basis for Misaminoacylation by Mutant E. Coli Glutaminyl-tRNA Synthetase Enzymes Authors: Perona, J.J. / Swanson, R.N. / Rould, M.A. / Steitz, T.A. / Soll, D. #3:  Journal: Science / Year: 1989 Journal: Science / Year: 1989Title: Structure of E. Coli Glutaminyl-tRNA Synthetase Complexed with tRNA(Gln) and ATP at 2.8 A Resolution Authors: Rould, M.A. / Perona, J.J. / Soll, D. / Steitz, T.A. #4:  Journal: Proc.Natl.Acad.Sci.USA / Year: 1984 Journal: Proc.Natl.Acad.Sci.USA / Year: 1984Title: Transfer RNA Mischarging Mediated by a Mutant Escherichia Coli Glutaminyl-tRNA Synthetase Authors: Inokuchi, H. / Hoben, P. / Yamao, F. / Ozeki, H. / Soll, D. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1qrs.cif.gz 1qrs.cif.gz | 181.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1qrs.ent.gz pdb1qrs.ent.gz | 147.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1qrs.json.gz 1qrs.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qr/1qrs https://data.pdbj.org/pub/pdb/validation_reports/qr/1qrs ftp://data.pdbj.org/pub/pdb/validation_reports/qr/1qrs ftp://data.pdbj.org/pub/pdb/validation_reports/qr/1qrs | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: RNA chain | Mass: 24060.287 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli (E. coli) / Strain: K-12 / Description: LAMBDA PL PROMOTER / Gene: TRNAGLN2 / Variant: DELTAH1DELTATRP / Plasmid details: PLC28 DERIVATIVE / Plasmid: PRS3 (PLC28 DERIVATIVE) / Gene (production host): TRNAGLN2 / Production host: Escherichia coli (E. coli) / Strain: K-12 / Description: LAMBDA PL PROMOTER / Gene: TRNAGLN2 / Variant: DELTAH1DELTATRP / Plasmid details: PLC28 DERIVATIVE / Plasmid: PRS3 (PLC28 DERIVATIVE) / Gene (production host): TRNAGLN2 / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
|---|---|
| #2: Protein | Mass: 63433.648 Da / Num. of mol.: 1 / Mutation: D235N Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli (E. coli) / Strain: X3R2 (GLNS DELETION STRAIN) / Description: GLNS PROMOTER (NO OVEREXPRESSION) / Gene: GLNS7 / Plasmid details: DERIVATIVE OF PBR / Plasmid: PYY140 (DERIVATIVE OF PBR322) / Gene (production host): GLNS7 / Production host: Escherichia coli (E. coli) / Strain: X3R2 (GLNS DELETION STRAIN) / Description: GLNS PROMOTER (NO OVEREXPRESSION) / Gene: GLNS7 / Plasmid details: DERIVATIVE OF PBR / Plasmid: PYY140 (DERIVATIVE OF PBR322) / Gene (production host): GLNS7 / Production host:   Escherichia coli (E. coli) / Escherichia coli (E. coli) /  Keywords: MUTANT D235N / References: UniProt: P00962, Keywords: MUTANT D235N / References: UniProt: P00962,  glutamine-tRNA ligase glutamine-tRNA ligase |
| #3: Chemical | ChemComp-ATP /  Adenosine triphosphate Adenosine triphosphate |
| #4: Water | ChemComp-HOH /  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 3 X-RAY DIFFRACTION / Number of used crystals: 3 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.78 Å3/Da / Density % sol: 70 % | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal | *PLUS Density % sol: 70 % | ||||||||||||||||||||||||||||||||||||||||||
Crystal grow | *PLUS Temperature: 17 ℃ / Method: vapor diffusion, hanging drop / PH range low: 7.5 / PH range high: 7 | ||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Detector | Type: SDMS / Detector: AREA DETECTOR / Date: Dec 30, 1990 |
|---|---|
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Relative weight: 1 |
| Reflection | Num. obs: 36285 / % possible obs: 91.4 % / Observed criterion σ(I): 0 / Redundancy: 3.3 % / Rmerge(I) obs: 0.047 |
| Reflection | *PLUS Highest resolution: 2.6 Å / Lowest resolution: 8 Å / % possible obs: 91.4 % / Observed criterion σ(I): 0 / Redundancy: 3.3 % |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.6→6 Å / σ(F): 0 /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 31.25 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.4 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.6→6 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Classification: refinement X-PLOR / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2.6 Å / Lowest resolution: 6 Å / σ(F): 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS |
 Movie
Movie Controller
Controller










 PDBj
PDBj
































