+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1qr4 | ||||||
|---|---|---|---|---|---|---|---|
| Title | TWO FIBRONECTIN TYPE-III DOMAIN SEGMENT FROM CHICKEN TENASCIN | ||||||
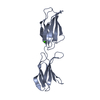
 Components Components | PROTEIN (TENASCIN) | ||||||
 Keywords Keywords |  STRUCTURAL PROTEIN / STRUCTURAL PROTEIN /  TENASCIN / FIBRONECTIN TYPE-III / TENASCIN / FIBRONECTIN TYPE-III /  HEPARIN / HEPARIN /  EXTRACELLULAR MATRIX / EXTRACELLULAR MATRIX /  ADHESION / ADHESION /  FUSION PROTEIN FUSION PROTEIN | ||||||
| Function / homology |  Function and homology information Function and homology informationcollagen-containing extracellular matrix /  cell adhesion / cell adhesion /  signaling receptor binding / signaling receptor binding /  extracellular space extracellular spaceSimilarity search - Function | ||||||
| Biological species |   Gallus gallus (chicken) Gallus gallus (chicken) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.55 Å MOLECULAR REPLACEMENT / Resolution: 2.55 Å | ||||||
 Authors Authors | Piontek, K. / Bisig, D.A. | ||||||
 Citation Citation |  Journal: Acta Crystallogr.,Sect.D / Year: 1999 Journal: Acta Crystallogr.,Sect.D / Year: 1999Title: Purification, crystallization and preliminary crystallographic studies of a two fibronectin type-III domain segment from chicken tenascin encompassing the heparin- and contactin-binding regions. Authors: Bisig, D. / Weber, P. / Vaughan, L. / Winterhalter, K.H. / Piontek, K. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1qr4.cif.gz 1qr4.cif.gz | 81 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1qr4.ent.gz pdb1qr4.ent.gz | 60.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1qr4.json.gz 1qr4.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qr/1qr4 https://data.pdbj.org/pub/pdb/validation_reports/qr/1qr4 ftp://data.pdbj.org/pub/pdb/validation_reports/qr/1qr4 ftp://data.pdbj.org/pub/pdb/validation_reports/qr/1qr4 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1fnfS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| 2 | 
| ||||||||||||
| Unit cell |
| ||||||||||||
| Noncrystallographic symmetry (NCS) | NCS oper:
|
- Components
Components
| #1: Protein | Mass: 20260.840 Da / Num. of mol.: 2 / Fragment: FNIII DOMAINS 5-6 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Gallus gallus (chicken) / Cellular location: EXTRACELLULAR MATRIX Gallus gallus (chicken) / Cellular location: EXTRACELLULAR MATRIX / Plasmid: PDS9/56 / Cellular location (production host): CYTOPLASM / Production host: / Plasmid: PDS9/56 / Cellular location (production host): CYTOPLASM / Production host:   Escherichia coli (E. coli) / Strain (production host): M15 / References: UniProt: P10039 Escherichia coli (E. coli) / Strain (production host): M15 / References: UniProt: P10039#2: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 2 X-RAY DIFFRACTION / Number of used crystals: 2 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.3 Å3/Da / Density % sol: 47 % Description: DATA WERE COLLECTED AT THE SYNCHROTRON FACILITIES OF DESY/X31 AND ESRF/BM01A | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Crystal grow | pH: 5 Details: 27% PEG2000, 100MM NAAC PH 4.5-5.0, 10-100 MM MGCL2/CACL2, | ||||||||||||||||||||||||||||||
| Crystal | *PLUS | ||||||||||||||||||||||||||||||
| Crystal grow | *PLUS pH: 4.5 / Method: vapor diffusion, hanging drop / Details: used to seeding | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 277 K |
|---|---|
| Diffraction source | Wavelength: 0.9 |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.9 Å / Relative weight: 1 : 0.9 Å / Relative weight: 1 |
| Reflection | Resolution: 2.55→20 Å / Num. obs: 11344 / % possible obs: 94 % / Redundancy: 6 % / Rmerge(I) obs: 0.074 / Rsym value: 0.074 / Net I/σ(I): 13 |
| Reflection shell | Resolution: 2.55→2.64 Å / Rmerge(I) obs: 0.47 / Mean I/σ(I) obs: 2 / Rsym value: 0.47 / % possible all: 70.1 |
| Reflection | *PLUS Num. measured all: 68031 |
| Reflection shell | *PLUS % possible obs: 75.5 % / Mean I/σ(I) obs: 2.4 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1FNF Resolution: 2.55→17.8 Å / Data cutoff high absF: 1000000 / Data cutoff low absF: 0 / Cross valid method: THROUGHOUT / σ(F): 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 49.1 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.55→17.8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints NCS | NCS model details: RESTRAINTS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.55→2.67 Å / Total num. of bins used: 8 /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.851 / Classification: refinement X-PLOR / Version: 3.851 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Lowest resolution: 17.8 Å / σ(F): 0 / % reflection Rfree: 10 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS Biso mean: 49.1 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor Rfree: 0.421 / Rfactor Rwork: 0.377 |
 Movie
Movie Controller
Controller












 PDBj
PDBj








