[English] 日本語
 Yorodumi
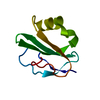
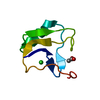
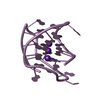
Yorodumi- PDB-1ptx: CRYSTAL STRUCTURE OF TOXIN II FROM THE SCORPION ANDROCTONUS AUSTR... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1ptx | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF TOXIN II FROM THE SCORPION ANDROCTONUS AUSTRALIS HECTOR REFINED AT 1.3 ANGSTROMS RESOLUTION | ||||||
 Components Components | SCORPION TOXIN II | ||||||
 Keywords Keywords |  TOXIN TOXIN | ||||||
| Function / homology |  Function and homology information Function and homology information sodium channel inhibitor activity / defense response / sodium channel inhibitor activity / defense response /  toxin activity / extracellular region toxin activity / extracellular regionSimilarity search - Function | ||||||
| Biological species |   Androctonus australis (Sahara scorpion) Androctonus australis (Sahara scorpion) | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 1.3 Å X-RAY DIFFRACTION / Resolution: 1.3 Å | ||||||
 Authors Authors | Fontecilla-Camps, J.C. / Housset, D. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 1994 Journal: J.Mol.Biol. / Year: 1994Title: Crystal structure of toxin II from the scorpion Androctonus australis Hector refined at 1.3 A resolution. Authors: Housset, D. / Habersetzer-Rochat, C. / Astier, J.P. / Fontecilla-Camps, J.C. #1:  Journal: J.Mol.Evol. / Year: 1989 Journal: J.Mol.Evol. / Year: 1989Title: Three-Dimensional Model of the Insect-Directed Scorpion Toxin from Androctonus Australis Hector and its Implication for the Evolution of Scorpion Toxins in General Authors: Fontecilla-Camps, J.C. #2:  Journal: Proc.Natl.Acad.Sci.USA / Year: 1988 Journal: Proc.Natl.Acad.Sci.USA / Year: 1988Title: Orthorhombic Crystals and Three-Dimensional Structure of the Potent Toxin II from the Scorpion Androctonus Australis Hector Authors: Fontecilla-Camps, J.C. / Habersetzer-Rochat, C. / Rochat, H. #3:  Journal: Proc.Natl.Acad.Sci.USA / Year: 1980 Journal: Proc.Natl.Acad.Sci.USA / Year: 1980Title: Three-Dimensional Structure of a Protein from Scorpion Venom: A New Structural Class of Neurotoxin Authors: Fontecilla-Camps, J.C. / Almassy, R.J. / Suddath, F.L. / Watt, D.D. / Bugg, C.E. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1ptx.cif.gz 1ptx.cif.gz | 22.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1ptx.ent.gz pdb1ptx.ent.gz | 17 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1ptx.json.gz 1ptx.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pt/1ptx https://data.pdbj.org/pub/pdb/validation_reports/pt/1ptx ftp://data.pdbj.org/pub/pdb/validation_reports/pt/1ptx ftp://data.pdbj.org/pub/pdb/validation_reports/pt/1ptx | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein |  Mass: 7262.167 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Androctonus australis (Sahara scorpion) Androctonus australis (Sahara scorpion)References: UniProt: P01484 |
|---|---|
| #2: Water | ChemComp-HOH /  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.92 Å3/Da / Density % sol: 36.09 % | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Crystal grow | *PLUS Temperature: 4 ℃ / pH: 6.8 / Method: seeding | ||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| Reflection | *PLUS Highest resolution: 1.3 Å / Lowest resolution: 9999 Å / Num. all: 14393 / Num. obs: 13619 / % possible obs: 94.6 % / Rmerge(I) obs: 0.12 |
| Reflection shell | *PLUS Highest resolution: 1.3 Å / Lowest resolution: 1.32 Å / % possible obs: 86.1 % / Num. possible: 693 / Num. unique obs: 597 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.3→7 Å / σ(F): 2.5 /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.3→7 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: X-PLOR/PROLSQ / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor obs: 0.148 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller









 PDBj
PDBj


