+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1kq8 | ||||||
|---|---|---|---|---|---|---|---|
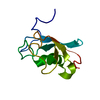
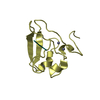
| Title | Solution Structure of Winged Helix Protein HFH-1 | ||||||
 Components Components | HEPATOCYTE NUCLEAR FACTOR 3 FORKHEAD HOMOLOG 1 | ||||||
 Keywords Keywords |  TRANSCRIPTION / TRANSCRIPTION /  Winged Helix Protein / HFH-1 Winged Helix Protein / HFH-1 | ||||||
| Function / homology |  Function and homology information Function and homology informationhair follicle morphogenesis / anatomical structure morphogenesis / DNA-binding transcription repressor activity, RNA polymerase II-specific / negative regulation of neuron apoptotic process /  cell differentiation / DNA-binding transcription factor activity, RNA polymerase II-specific / RNA polymerase II cis-regulatory region sequence-specific DNA binding / regulation of transcription by RNA polymerase II / negative regulation of transcription by RNA polymerase II / cell differentiation / DNA-binding transcription factor activity, RNA polymerase II-specific / RNA polymerase II cis-regulatory region sequence-specific DNA binding / regulation of transcription by RNA polymerase II / negative regulation of transcription by RNA polymerase II /  nucleus nucleusSimilarity search - Function | ||||||
| Biological species |   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) | ||||||
| Method |  SOLUTION NMR / torsion angle dynamics SOLUTION NMR / torsion angle dynamics | ||||||
 Authors Authors | Sheng, W. / Rance, M. / Liao, X. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2002 Journal: Biochemistry / Year: 2002Title: Structure comparison of two conserved HNF-3/fkh proteins HFH-1 and genesis indicates the existence of folding differences in their complexes with a DNA binding sequence. Authors: Sheng, W. / Rance, M. / Liao, X. #1:  Journal: J.Mol.Biol. / Year: 1998 Journal: J.Mol.Biol. / Year: 1998Title: Structural changes in the region directly adjacent to the DNA-binding helix highlight a possible mechanism to explain the observed changes in the sequence-specific binding of winged helix proteins Authors: Marsden, I. / Jin, C. / Liao, X. #2:  Journal: Biochemistry / Year: 1998 Journal: Biochemistry / Year: 1998Title: Sequence specific collective motions in a winged helix DNA binding domain detected by 15N relaxation NMR Authors: Jin, C. / Marsden, I. / Chen, X. / Liao, X. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1kq8.cif.gz 1kq8.cif.gz | 478.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1kq8.ent.gz pdb1kq8.ent.gz | 413.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1kq8.json.gz 1kq8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/kq/1kq8 https://data.pdbj.org/pub/pdb/validation_reports/kq/1kq8 ftp://data.pdbj.org/pub/pdb/validation_reports/kq/1kq8 ftp://data.pdbj.org/pub/pdb/validation_reports/kq/1kq8 | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 11804.493 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Rattus norvegicus (Norway rat) / Production host: Rattus norvegicus (Norway rat) / Production host:   Escherichia coli (E. coli) / References: UniProt: Q63244 Escherichia coli (E. coli) / References: UniProt: Q63244 |
|---|
-Experimental details
-Experiment
| Experiment | Method:  SOLUTION NMR SOLUTION NMR | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Sample conditions | pH: 6.5 / Temperature: 290 K |
|---|---|
Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| NMR spectrometer |
|
|---|
- Processing
Processing
| NMR software | Name: DYANA / Version: 1.5 / Developer: Gunter, P. / Classification: refinement |
|---|---|
| Refinement | Method: torsion angle dynamics / Software ordinal: 1 |
| NMR ensemble | Conformer selection criteria: target function / Conformers calculated total number: 200 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller











 PDBj
PDBj