+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1jbi | ||||||
|---|---|---|---|---|---|---|---|
| Title | NMR structure of the LCCL domain | ||||||
 Components Components | cochlin | ||||||
 Keywords Keywords |  STRUCTURAL GENOMICS / UNKNOWN FUNCTION / STRUCTURAL GENOMICS / UNKNOWN FUNCTION /  alpha-beta protein alpha-beta protein | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of innate immune response /  collagen binding / sensory perception of sound / regulation of cell shape / collagen-containing extracellular matrix / defense response to bacterium collagen binding / sensory perception of sound / regulation of cell shape / collagen-containing extracellular matrix / defense response to bacteriumSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  SOLUTION NMR / torsion angle dynamics,simulated annealing SOLUTION NMR / torsion angle dynamics,simulated annealing | ||||||
 Authors Authors | Liepinsh, E. / Trexler, M. / Kaikkonen, A. / Weigelt, J. / Banyai, L. / Patthy, L. / Otting, G. | ||||||
 Citation Citation |  Journal: EMBO J. / Year: 2001 Journal: EMBO J. / Year: 2001Title: NMR structure of the LCCL domain and implications for DFNA9 deafness disorder. Authors: Liepinsh, E. / Trexler, M. / Kaikkonen, A. / Weigelt, J. / Banyai, L. / Patthy, L. / Otting, G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1jbi.cif.gz 1jbi.cif.gz | 569.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1jbi.ent.gz pdb1jbi.ent.gz | 494.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1jbi.json.gz 1jbi.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jb/1jbi https://data.pdbj.org/pub/pdb/validation_reports/jb/1jbi ftp://data.pdbj.org/pub/pdb/validation_reports/jb/1jbi ftp://data.pdbj.org/pub/pdb/validation_reports/jb/1jbi | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 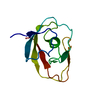
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 10609.979 Da / Num. of mol.: 1 / Fragment: LCCL module Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: M13mp18 / Plasmid: pmed23 / Production host: Homo sapiens (human) / Gene: M13mp18 / Plasmid: pmed23 / Production host:   Escherichia coli (E. coli) / Strain (production host): JM 109 / References: UniProt: O43405 Escherichia coli (E. coli) / Strain (production host): JM 109 / References: UniProt: O43405 |
|---|
-Experimental details
-Experiment
| Experiment | Method:  SOLUTION NMR SOLUTION NMR | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
| ||||||||||||
| NMR details | Text: This structure was determined using standard 2D homonuclear techniques |
- Sample preparation
Sample preparation
| Details |
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample conditions |
| |||||||||||||||
Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radiation wavelength | Relative weight: 1 | |||||||||||||||
| NMR spectrometer |
|
- Processing
Processing
| NMR software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: torsion angle dynamics,simulated annealing / Software ordinal: 1 Details: the structures are based on a total of 2532 restraints, 1193 are NOE-derived distance constraints, 270 dihedral angle restraints and 69 residual dipolar coupling restraints | ||||||||||||||||||||||||
| NMR representative | Selection criteria: closest to the average | ||||||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: structures with the lowest energy,target function Conformers calculated total number: 50 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller











 PDBj
PDBj


