[English] 日本語
 Yorodumi
Yorodumi- PDB-2htf: The solution structure of the BRCT domain from human polymerase r... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2htf | ||||||
|---|---|---|---|---|---|---|---|
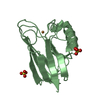
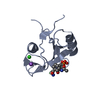
| Title | The solution structure of the BRCT domain from human polymerase reveals homology with the TdT BRCT domain | ||||||
 Components Components | DNA polymerase mu | ||||||
 Keywords Keywords |  TRANSFERASE / TRANSFERASE /  BRCT domain / alpha-beta-alpha sandwich BRCT domain / alpha-beta-alpha sandwich | ||||||
| Function / homology |  Function and homology information Function and homology informationNonhomologous End-Joining (NHEJ) / double-strand break repair via nonhomologous end joining / DNA recombination /  DNA-directed DNA polymerase / DNA-directed DNA polymerase /  DNA-directed DNA polymerase activity / DNA-directed DNA polymerase activity /  DNA binding / DNA binding /  nucleoplasm / nucleoplasm /  metal ion binding / metal ion binding /  nucleus nucleusSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  SOLUTION NMR / Torsion angle dynamics, molecular dynamics refinement in explicit water solvent SOLUTION NMR / Torsion angle dynamics, molecular dynamics refinement in explicit water solvent | ||||||
| Model type details | minimized average | ||||||
 Authors Authors | DeRose, E.F. / Clarkson, M.W. / Gilmore, S.A. / Ramsden, D.A. / Mueller, G.A. / London, R.E. / Lee, A.L. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2007 Journal: Biochemistry / Year: 2007Title: Solution structure of polymerase mu's BRCT Domain reveals an element essential for its role in nonhomologous end joining. Authors: DeRose, E.F. / Clarkson, M.W. / Gilmore, S.A. / Galban, C.J. / Tripathy, A. / Havener, J.M. / Mueller, G.A. / Ramsden, D.A. / London, R.E. / Lee, A.L. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2htf.cif.gz 2htf.cif.gz | 380.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2htf.ent.gz pdb2htf.ent.gz | 332.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2htf.json.gz 2htf.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ht/2htf https://data.pdbj.org/pub/pdb/validation_reports/ht/2htf ftp://data.pdbj.org/pub/pdb/validation_reports/ht/2htf ftp://data.pdbj.org/pub/pdb/validation_reports/ht/2htf | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein |  / Pol Mu / Pol MuMass: 11420.022 Da / Num. of mol.: 1 / Fragment: BRCT domain / Mutation: S20G Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: POLM / Production host: Homo sapiens (human) / Gene: POLM / Production host:   Escherichia coli (E. coli) / References: UniProt: Q9NP87, Escherichia coli (E. coli) / References: UniProt: Q9NP87,  DNA-directed DNA polymerase DNA-directed DNA polymerase |
|---|
-Experimental details
-Experiment
| Experiment | Method:  SOLUTION NMR SOLUTION NMR | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Details | Contents: 1mM polymerase mu BRCT domain, 25 mM d-Tris, 50 mM KCl, 0.02% NaN3, 5 mM DTT, pH 7.9, 90% H2O, 10% D2O Solvent system: 90% H2O/10% D2O |
|---|---|
| Sample conditions | Ionic strength: 50 mM KCl / pH: 7.9 / Pressure: ambient / Temperature: 283 K |
-NMR measurement
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radiation wavelength | Relative weight: 1 | |||||||||||||||
| NMR spectrometer |
|
- Processing
Processing
| NMR software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: Torsion angle dynamics, molecular dynamics refinement in explicit water solvent Software ordinal: 1 Details: The initial structures were computed using the auto noeassign feature of CYANA 2.1; 1503 NOE distance restraints were assigned, and 147 dihedral angle restraints were used in the calculation. ...Details: The initial structures were computed using the auto noeassign feature of CYANA 2.1; 1503 NOE distance restraints were assigned, and 147 dihedral angle restraints were used in the calculation. The 20 best CYANA structures were refined in explicit solvent using ARIA 2.0a with the 1506 NOE distance restraints, 147 dihedral angle restraints, 42 H-bond restraints, and 48 residual dipolar coupling restraints. | ||||||||||||||||||||||||
| NMR representative | Selection criteria: minimized average structure | ||||||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: structures with the lowest energy Conformers calculated total number: 100 / Conformers submitted total number: 11 |
 Movie
Movie Controller
Controller










 PDBj
PDBj



