+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-10346 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
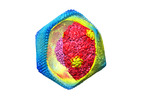
| タイトル | Structure of African Swine fever virus | ||||||||||||
 マップデータ マップデータ | Postprocessed map | ||||||||||||
 試料 試料 |
| ||||||||||||
| 生物種 |  African swine fever virus BA71V (ウイルス) African swine fever virus BA71V (ウイルス) | ||||||||||||
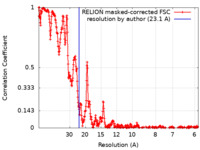
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 23.1 Å | ||||||||||||
 データ登録者 データ登録者 | Abrescia NG / Andres G / Charro D / Matamoros T / Dillard R | ||||||||||||
| 資金援助 |  スペイン, 3件 スペイン, 3件
| ||||||||||||
 引用 引用 |  ジャーナル: J Biol Chem / 年: 2020 ジャーナル: J Biol Chem / 年: 2020タイトル: The cryo-EM structure of African swine fever virus unravels a unique architecture comprising two icosahedral protein capsids and two lipoprotein membranes. 著者: German Andrés / Diego Charro / Tania Matamoros / Rebecca S Dillard / Nicola G A Abrescia /   要旨: African swine fever virus (ASFV) is a complex nucleocytoplasmic large DNA virus (NCLDV) that causes a devastating swine disease currently present in many countries of Africa, Europe, and Asia. ...African swine fever virus (ASFV) is a complex nucleocytoplasmic large DNA virus (NCLDV) that causes a devastating swine disease currently present in many countries of Africa, Europe, and Asia. Despite intense research efforts, relevant gaps in the architecture of the infectious virus particle remain. Here, we used single-particle cryo-EM to analyze the three-dimensional structure of the mature ASFV particle. Our results show that the ASFV virion, with a radial diameter of ∼2,080 Å, encloses a genome-containing nucleoid surrounded by two distinct icosahedral protein capsids and two lipoprotein membranes. The outer capsid forms a hexagonal lattice (triangulation number = 277) composed of 8,280 copies of the double jelly-roll major capsid protein (MCP) p72, arranged in trimers displaying a pseudo-hexameric morphology, and of 60 copies of a penton protein at the vertices. The inner protein layer, organized as a = 19 capsid, confines the core shell, and it is composed of the mature products derived from the ASFV polyproteins pp220 and pp62. Also, an icosahedral membrane lies between the two protein layers, whereas a pleomorphic envelope wraps the outer capsid. This high-level organization confers to ASFV a unique architecture among the NCLDVs that likely reflects the complexity of its infection process and may help explain current challenges in controlling it. | ||||||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_10346.map.gz emd_10346.map.gz | 3.4 GB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-10346-v30.xml emd-10346-v30.xml emd-10346.xml emd-10346.xml | 20.9 KB 20.9 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_10346_fsc.xml emd_10346_fsc.xml | 35.7 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_10346.png emd_10346.png | 515.1 KB | ||
| マスクデータ |  emd_10346_msk_1.map emd_10346_msk_1.map | 3.7 GB |  マスクマップ マスクマップ | |
| その他 |  emd_10346_additional_1.map.gz emd_10346_additional_1.map.gz emd_10346_additional_2.map.gz emd_10346_additional_2.map.gz emd_10346_half_map_1.map.gz emd_10346_half_map_1.map.gz emd_10346_half_map_2.map.gz emd_10346_half_map_2.map.gz | 504.4 MB 634.1 MB 3.4 GB 3.4 GB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10346 http://ftp.pdbj.org/pub/emdb/structures/EMD-10346 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10346 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10346 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_10346_validation.pdf.gz emd_10346_validation.pdf.gz | 410.4 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_10346_full_validation.pdf.gz emd_10346_full_validation.pdf.gz | 409.5 KB | 表示 | |
| XML形式データ |  emd_10346_validation.xml.gz emd_10346_validation.xml.gz | 35.4 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10346 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10346 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10346 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10346 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_10346.map.gz / 形式: CCP4 / 大きさ: 824 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_10346.map.gz / 形式: CCP4 / 大きさ: 824 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Postprocessed map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 2.938 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
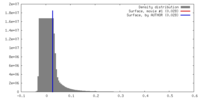
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
-マスク #1
| ファイル |  emd_10346_msk_1.map emd_10346_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
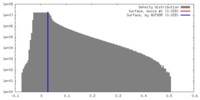
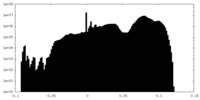
| 密度ヒストグラム |
-追加マップ: nucleocapsid
| ファイル | emd_10346_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | nucleocapsid | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
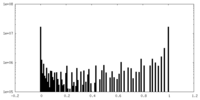
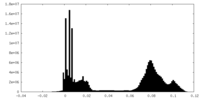
| 密度ヒストグラム |
-追加マップ: simmetrized nucleocapsid
| ファイル | emd_10346_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | simmetrized nucleocapsid | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
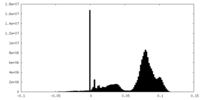
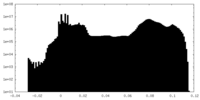
| 密度ヒストグラム |
-ハーフマップ: Half 2
| ファイル | emd_10346_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Half 2 | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-ハーフマップ: Half 1
| ファイル | emd_10346_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Half 1 | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
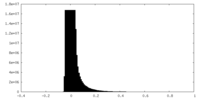
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : African swine fever virus BA71V
| 全体 | 名称:  African swine fever virus BA71V (ウイルス) African swine fever virus BA71V (ウイルス) |
|---|---|
| 要素 |
|
-超分子 #1: African swine fever virus BA71V
| 超分子 | 名称: African swine fever virus BA71V / タイプ: virus / ID: 1 / 親要素: 0 / 含まれる分子: #1 / NCBI-ID: 10498 / 生物種: African swine fever virus BA71V / ウイルスタイプ: VIRION / ウイルス・単離状態: STRAIN / ウイルス・エンベロープ: Yes / ウイルス・中空状態: No |
|---|---|
| 宿主 | 生物種: pigs, warthogs, bushpigs (unknown) |
| ウイルス殻 | Shell ID: 1 / 名称: Outer capsid (MCP p72) / 直径: 2400.0 Å / T番号(三角分割数): 277 |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 1.1 mg/mL |
|---|---|
| 緩衝液 | pH: 7.4 / 構成要素 - 式: PBS |
| グリッド | モデル: Quantifoil R2/2 / 材質: COPPER / メッシュ: 200 / 前処理 - タイプ: GLOW DISCHARGE |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / チャンバー内温度: 277 K / 装置: FEI VITROBOT MARK III |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 詳細 | Two datasets were acquired. |
| 撮影 | #0 - Image recording ID: 1 #0 - フィルム・検出器のモデル: FEI FALCON III (4k x 4k) #0 - 平均電子線量: 47.7 e/Å2 #0 - 詳細: Titan Krios equipped with a Cs-corrector and a Falcon 3EC direct electron detector #1 - Image recording ID: 2 #1 - フィルム・検出器のモデル: GATAN K2 SUMMIT (4k x 4k) #1 - 平均電子線量: 47.5 e/Å2 #1 - 詳細: Titan Krios equipped with a Gatan K2 Summit direct electron detector |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: DARK FIELD |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)