[English] 日本語
 Yorodumi
Yorodumi- EMDB-3462: negative-stain 3D reconstruction of Sso heterotrimeric holo DNA-PolB1 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3462 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | negative-stain 3D reconstruction of Sso heterotrimeric holo DNA-PolB1 | |||||||||
 Map data Map data | Final map for holo-PolB1* - no masked.Contoured in Chimera at 0.03 threshold. FSC have been calculated for this map at convergence. | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Archaea (unknown) Archaea (unknown) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  negative staining / Resolution: 20.2 Å negative staining / Resolution: 20.2 Å | |||||||||
 Authors Authors | Abrescia NGA / Bell SD | |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2017 Journal: Nat Commun / Year: 2017Title: Identification and characterization of a heterotrimeric archaeal DNA polymerase holoenzyme. Authors: Jiangyu Yan / Thomas R Beattie / Adriana L Rojas / Kelly Schermerhorn / Tamzin Gristwood / Jonathan C Trinidad / Sonja V Albers / Pietro Roversi / Andrew F Gardner / Nicola G A Abrescia / Stephen D Bell /     Abstract: Since their initial characterization over 30 years ago, it has been believed that the archaeal B-family DNA polymerases are single-subunit enzymes. This contrasts with the multi-subunit B-family ...Since their initial characterization over 30 years ago, it has been believed that the archaeal B-family DNA polymerases are single-subunit enzymes. This contrasts with the multi-subunit B-family replicative polymerases of eukaryotes. Here we reveal that the highly studied PolB1 from Sulfolobus solfataricus exists as a heterotrimeric complex in cell extracts. Two small subunits, PBP1 and PBP2, associate with distinct surfaces of the larger catalytic subunit and influence the enzymatic properties of the DNA polymerase. Thus, multi-subunit replicative DNA polymerase holoenzymes are present in all three domains of life. We reveal the architecture of the assembly by a combination of cross-linking coupled with mass spectrometry, X-ray crystallography and single-particle electron microscopy. The small subunits stabilize the holoenzyme assembly and the acidic tail of one small subunit mitigates the ability of the enzyme to perform strand-displacement synthesis, with important implications for lagging strand DNA synthesis. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3462.map.gz emd_3462.map.gz | 6.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3462-v30.xml emd-3462-v30.xml emd-3462.xml emd-3462.xml | 22 KB 22 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_3462.png emd_3462.png | 135 KB | ||
| Others |  emd_3462_half_map_1.map.gz emd_3462_half_map_1.map.gz emd_3462_half_map_2.map.gz emd_3462_half_map_2.map.gz | 6.1 MB 6.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3462 http://ftp.pdbj.org/pub/emdb/structures/EMD-3462 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3462 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3462 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_3462.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3462.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Final map for holo-PolB1* - no masked.Contoured in Chimera at 0.03 threshold. FSC have been calculated for this map at convergence. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.66 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
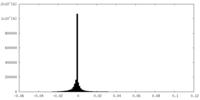
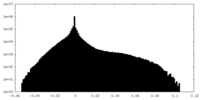
-Half map: half1 map for holo-PolB1*. Contoured in Chimera at 0.028 threshold
| File | emd_3462_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half1 map for holo-PolB1*. Contoured in Chimera at 0.028 threshold | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
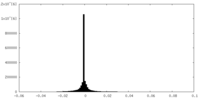
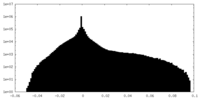
-Half map: half2 map for holo-PolB1*. Contoured in Chimera at 0.028 threshold
| File | emd_3462_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half2 map for holo-PolB1*. Contoured in Chimera at 0.028 threshold | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : archaeal heterotrimeric DNA polymerase B1 holo-complex
| Entire | Name: archaeal heterotrimeric DNA polymerase B1 holo-complex |
|---|---|
| Components |
|
-Supramolecule #1: archaeal heterotrimeric DNA polymerase B1 holo-complex
| Supramolecule | Name: archaeal heterotrimeric DNA polymerase B1 holo-complex type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Archaea (unknown) Archaea (unknown) |
| Recombinant expression | Organism:   Escherichia coli (E. coli) / Recombinant plasmid: pET33b/pET30a Escherichia coli (E. coli) / Recombinant plasmid: pET33b/pET30a |
| Molecular weight | Theoretical: 130 KDa |
-Macromolecule #1: Sulfolobus solfataricus DNA polymerase B1 enzime holo-complex
| Macromolecule | Name: Sulfolobus solfataricus DNA polymerase B1 enzime holo-complex type: protein_or_peptide / ID: 1 Details: The sample sequence contemplates the DNA polB1 but complex investigated by negative stain EM is formed by the PBP1, PBP2 and DNA. > DNA-oligo1 fragment ACAGGTAAGCAGTCCGCG > DNA-oligo2 fragment GCGGACTGCTTACDDC Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Archaea (unknown) Archaea (unknown) |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MAKQLTLFD IPSSKPAKSE QNTQQSQQSA PVEEKKVVRR EWLEEAQENK IYFLLQVDYD GKKGKAVCKL FDKETQKIYA LYDNTGHKPY FLVDLEPDKV GKIPKIVRDP SFDHIETVSK IDPYTWNKFK LTKIVVRDPL AVRRLRNDVP KAYEAHIKYF NNYMYDIGLI ...String: MAKQLTLFD IPSSKPAKSE QNTQQSQQSA PVEEKKVVRR EWLEEAQENK IYFLLQVDYD GKKGKAVCKL FDKETQKIYA LYDNTGHKPY FLVDLEPDKV GKIPKIVRDP SFDHIETVSK IDPYTWNKFK LTKIVVRDPL AVRRLRNDVP KAYEAHIKYF NNYMYDIGLI PGMPYVVKNG KLESVYLSLD EKDVEEIKKA FADSDEMTRQ MAVDWLPIFE TEIPKIKRVA IDIEVYTPVK GRIPDSQKAE FPIISIALAG SDGLKKVLVL NRNDVNEGSV KLDGISVERF NTEYELLGRF FDILLEYPIV LTFNGDDFDL PYIYFRALKL GYFPEEIPID VAGKDEAKYL AGLHIDLYKF FFNKAVRNYA FEGKYNEYNL DAVAKALLGT SKVKVDTLIS FLDVEKLIEY NFRDAEITLQ LTTFNNDLTM KLIVLFSRIS RLGIEELTRT EISTWVKNLY YWEHRKRNWL IPLKEEILAK SSNIRTSALI KGKGYKGAVV IDPPAGIFFN ITVLDFASLY PSIIRTWNLS YETVDIQQCK KPYEVKDETG EVLHIVCMDR PGITAVITGL LRDFRVKIYK KKAKNPNNSE EQKLLYDVVQ RAMKVFINAT YGVFGAETFP LYAPAVAESV TALGRYVITS TVKKAREEGL TVLYGDTDSL FLLNPPKNSL ENIIKWVKTT FNLDLEVDKT YKFVAFSGLK KNYFGVYQDG KVDIKGMLVK KRNTPEFVKK VFNEVKELMI SINSPNDVKE IKRKIVDVVK GSYEKLKNKG YNLDELAFKV MLSKPLDAYK KNTPQHVKAA LQLRPFGVNV LPRDIIYYVK VRSKDGVKPV QLAKVTEIDA EKYLEALRST FEQILRAFGV SWDEIAATMS IDSFFSYPSK GNS |
-Macromolecule #2: Polymerase Binding Protein 1 - delta CTD
| Macromolecule | Name: Polymerase Binding Protein 1 - delta CTD / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Archaea (unknown) Archaea (unknown) |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: ISHMSTRWLP KWKAIEIDYN NKKVTVCYDE VTRLYVCPIC SPNCAKGVST DYSTYFFNLE DLKRHLDAHK YGLWLQKKTR T |
-Macromolecule #3: Polymerase Binding Protein 2
| Macromolecule | Name: Polymerase Binding Protein 2 / type: protein_or_peptide / ID: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Archaea (unknown) Archaea (unknown) |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MSVNQKEIEI AIEYFKNYIS VGEIVATMDL KARGISNPQA VISKLIEMGI IEKGEGCYNL VRKSTDKKle hhhhhh |
-Macromolecule #4: DNA fragment
| Macromolecule | Name: DNA fragment / type: dna / ID: 4 / Details: oligonucleotide primer-template junction / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Archaea (unknown) Archaea (unknown) |
| Sequence | String: ACAGGTAAGC AGTCCGCG G CGGACTGCTT ACDDC |
-Experimental details
-Structure determination
| Method |  negative staining negative staining |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.01 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||
| Staining | Type: NEGATIVE / Material: uranyl formate Details: Negatively stained EM specimens were prepared using carbon-coated grids and stained with 2% of uranyl formate solution. | ||||||||||||
| Grid | Model: EMS / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.02 kPa |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 2200FSC |
|---|---|
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 90201 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.0 mm / Nominal defocus max: 1.7 µm / Nominal defocus min: 1.3 µm / Nominal magnification: 60000 Bright-field microscopy / Cs: 2.0 mm / Nominal defocus max: 1.7 µm / Nominal defocus min: 1.3 µm / Nominal magnification: 60000 |
| Specialist optics | Energy filter - Name: In-column Omega Filter / Energy filter - Lower energy threshold: 0 eV / Energy filter - Upper energy threshold: 20 eV |
| Sample stage | Specimen holder model: JEOL |
| Image recording | Film or detector model: GATAN ULTRASCAN 4000 (4k x 4k) / Number real images: 400 / Average exposure time: 0.5 sec. / Average electron dose: 30.0 e/Å2 |
- Image processing
Image processing
| Particle selection | Number selected: 110000 |
|---|---|
| CTF correction | Software - Name: CTFFIND (ver. 3) / Details: phase flipping was performed using XMIPP software |
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: Details: The initial reference map was generated using the atomic model. |
| Initial angle assignment | Type: PROJECTION MATCHING / Software - Name: RELION (ver. 1.4) |
| Final angle assignment | Type: PROJECTION MATCHING Projection matching processing - Angular sampling: 3.75 degrees Software - Name: RELION / Software - details: RELION through SCIPION |
| Final reconstruction | Number classes used: 1 / Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 20.2 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: RELION / Software - details: RELION through SCIPION Details: Calculated for the converged iteration 13 in Relion using Scipion Number images used: 11758 |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: Cross-correlation coefficient |
|---|
 Movie
Movie Controller
Controller














 Z
Z Y
Y X
X