+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22222 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | SARS-CoV-2 HexaPro S Two RBD up | |||||||||
 Map data Map data | Full map sharpened | |||||||||
 Sample Sample |
| |||||||||
| Biological species |   Severe acute respiratory syndrome coronavirus 2 Severe acute respiratory syndrome coronavirus 2 | |||||||||
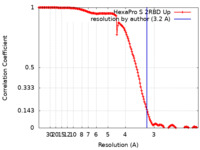
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.2 Å cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Wrapp D / Hsieh C-L / Goldsmith JA / McLellan JS | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation | Journal: bioRxiv / Year: 2020 Title: Structure-based Design of Prefusion-stabilized SARS-CoV-2 Spikes. Authors: Ching-Lin Hsieh / Jory A Goldsmith / Jeffrey M Schaub / Andrea M DiVenere / Hung-Che Kuo / Kamyab Javanmardi / Kevin C Le / Daniel Wrapp / Alison Gene-Wei Lee / Yutong Liu / Chia-Wei Chou / ...Authors: Ching-Lin Hsieh / Jory A Goldsmith / Jeffrey M Schaub / Andrea M DiVenere / Hung-Che Kuo / Kamyab Javanmardi / Kevin C Le / Daniel Wrapp / Alison Gene-Wei Lee / Yutong Liu / Chia-Wei Chou / Patrick O Byrne / Christy K Hjorth / Nicole V Johnson / John Ludes-Meyers / Annalee W Nguyen / Juyeon Park / Nianshuang Wang / Dzifa Amengor / Jennifer A Maynard / Ilya J Finkelstein / Jason S McLellan /  Abstract: The COVID-19 pandemic caused by the novel coronavirus SARS-CoV-2 has led to accelerated efforts to develop therapeutics, diagnostics, and vaccines to mitigate this public health emergency. A key ...The COVID-19 pandemic caused by the novel coronavirus SARS-CoV-2 has led to accelerated efforts to develop therapeutics, diagnostics, and vaccines to mitigate this public health emergency. A key target of these efforts is the spike (S) protein, a large trimeric class I fusion protein that is metastable and difficult to produce recombinantly in large quantities. Here, we designed and expressed over 100 structure-guided spike variants based upon a previously determined cryo-EM structure of the prefusion SARS-CoV-2 spike. Biochemical, biophysical and structural characterization of these variants identified numerous individual substitutions that increased protein yields and stability. The best variant, HexaPro, has six beneficial proline substitutions leading to ~10-fold higher expression than its parental construct and is able to withstand heat stress, storage at room temperature, and multiple freeze-thaws. A 3.2 Å-resolution cryo-EM structure of HexaPro confirmed that it retains the prefusion spike conformation. High-yield production of a stabilized prefusion spike protein will accelerate the development of vaccines and serological diagnostics for SARS-CoV-2. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22222.map.gz emd_22222.map.gz | 290.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22222-v30.xml emd-22222-v30.xml emd-22222.xml emd-22222.xml | 21.8 KB 21.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_22222_fsc.xml emd_22222_fsc.xml | 16.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_22222.png emd_22222.png | 139 KB | ||
| Others |  emd_22222_additional.map.gz emd_22222_additional.map.gz emd_22222_additional_1.map.gz emd_22222_additional_1.map.gz emd_22222_half_map_1.map.gz emd_22222_half_map_1.map.gz emd_22222_half_map_2.map.gz emd_22222_half_map_2.map.gz | 154.7 MB 154.7 MB 285.7 MB 285.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22222 http://ftp.pdbj.org/pub/emdb/structures/EMD-22222 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22222 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22222 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_22222.map.gz / Format: CCP4 / Size: 307.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22222.map.gz / Format: CCP4 / Size: 307.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Full map sharpened | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
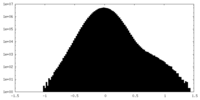
-Additional map: Full map unsharpened
| File | emd_22222_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Full map unsharpened | ||||||||||||
| Projections & Slices |
| ||||||||||||
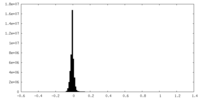
| Density Histograms |
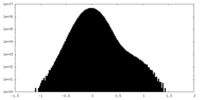
-Additional map: Full map unsharpened
| File | emd_22222_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Full map unsharpened | ||||||||||||
| Projections & Slices |
| ||||||||||||
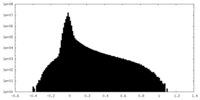
| Density Histograms |
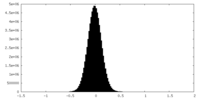
-Half map: Half-map A
| File | emd_22222_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
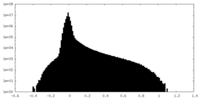
| Density Histograms |
-Half map: Half-map B
| File | emd_22222_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
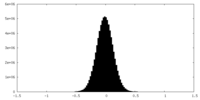
| Density Histograms |
- Sample components
Sample components
-Entire : Prefusion-stabilized SARS-CoV-2 spike trimer with two RBDs up
| Entire | Name: Prefusion-stabilized SARS-CoV-2 spike trimer with two RBDs up |
|---|---|
| Components |
|
-Supramolecule #1: Prefusion-stabilized SARS-CoV-2 spike trimer with two RBDs up
| Supramolecule | Name: Prefusion-stabilized SARS-CoV-2 spike trimer with two RBDs up type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Severe acute respiratory syndrome coronavirus 2 Severe acute respiratory syndrome coronavirus 2 |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Experimental: 600 KDa |
-Macromolecule #1: SARS-CoV-2 HexaPro S
| Macromolecule | Name: SARS-CoV-2 HexaPro S / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Severe acute respiratory syndrome coronavirus 2 Severe acute respiratory syndrome coronavirus 2 |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MFVFLVLLPL VSSQCVNLTT RTQLPPAYTN SFTRGVYYPD KVFRSSVLHS TQDLFLPFFS NVTWFHAIHV SGTNGTKRFD NPVLPFNDGV YFASTEKSNI IRGWIFGTTL DSKTQSLLIV NNATNVVIKV CEFQFCNDPF LGVYYHKNNK SWMESEFRVY SSANNCTFEY ...String: MFVFLVLLPL VSSQCVNLTT RTQLPPAYTN SFTRGVYYPD KVFRSSVLHS TQDLFLPFFS NVTWFHAIHV SGTNGTKRFD NPVLPFNDGV YFASTEKSNI IRGWIFGTTL DSKTQSLLIV NNATNVVIKV CEFQFCNDPF LGVYYHKNNK SWMESEFRVY SSANNCTFEY VSQPFLMDLE GKQGNFKNLR EFVFKNIDGY FKIYSKHTPI NLVRDLPQGF SALEPLVDLP IGINITRFQT LLALHRSYLT PGDSSSGWTA GAAAYYVGYL QPRTFLLKYN ENGTITDAVD CALDPLSETK CTLKSFTVEK GIYQTSNFRV QPTESIVRFP NITNLCPFGE VFNATRFASV YAWNRKRISN CVADYSVLYN SASFSTFKCY GVSPTKLNDL CFTNVYADSF VIRGDEVRQI APGQTGKIAD YNYKLPDDFT GCVIAWNSNN LDSKVGGNYN YLYRLFRKSN LKPFERDIST EIYQAGSTPC NGVEGFNCYF PLQSYGFQPT NGVGYQPYRV VVLSFELLHA PATVCGPKKS TNLVKNKCVN FNFNGLTGTG VLTESNKKFL PFQQFGRDIA DTTDAVRDPQ TLEILDITPC SFGGVSVITP GTNTSNQVAV LYQDVNCTEV PVAIHADQLT PTWRVYSTGS NVFQTRAGCL IGAEHVNNSY ECDIPIGAGI CASYQTQTNS PGSASSVASQ SIIAYTMSLG AENSVAYSNN SIAIPTNFTI SVTTEILPVS MTKTSVDCTM YICGDSTECS NLLLQYGSFC TQLNRALTGI AVEQDKNTQE VFAQVKQIYK TPPIKDFGGF NFSQILPDPS KPSKRSPIED LLFNKVTLAD AGFIKQYGDC LGDIAARDLI CAQKFNGLTV LPPLLTDEMI AQYTSALLAG TITSGWTFGA GPALQIPFPM QMAYRFNGIG VTQNVLYENQ KLIANQFNSA IGKIQDSLSS TPSALGKLQD VVNQNAQALN TLVKQLSSNF GAISSVLNDI LSRLDPPEAE VQIDRLITGR LQSLQTYVTQ QLIRAAEIRA SANLAATKMS ECVLGQSKRV DFCGKGYHLM SFPQSAPHGV VFLHVTYVPA QEKNFTTAPA ICHDGKAHFP REGVFVSNGT HWFVTQRNFY EPQIITTDNT FVSGNCDVVI GIVNNTVYDP LQPELDSFKE ELDKYFKNHT SPDVDLGDIS GINASVVNIQ KEIDRLNEVA KNLNESLIDL QELGKYEQGS GYIPEAPRDG QAYVRKDGEW VLLSTFLGRS LEVLFQGPGH HHHHHHHSAW SHPQFEKGGG SGGGGSGGSA WSHPQFEK |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.35 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 400 / Pretreatment - Type: PLASMA CLEANING | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy Bright-field microscopy |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 45.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z
Z Y
Y X
X