[English] 日本語
 Yorodumi
Yorodumi- PDB-5mdx: Cryo-EM structure of the PSII supercomplex from Arabidopsis thaliana -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5mdx | ||||||
|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the PSII supercomplex from Arabidopsis thaliana | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  PHOTOSYNTHESIS / PHOTOSYNTHESIS /  photosystem II supercomplex / photosystem II supercomplex /  single particle analysis single particle analysis | ||||||
| Function / homology |  Function and homology information Function and homology informationplastid thylakoid membrane / regulation of protein dephosphorylation /  photoinhibition / photoinhibition /  photosystem II antenna complex / PSII associated light-harvesting complex II / nonphotochemical quenching / sequestering of metal ion / chloroplast stromal thylakoid / photosystem II antenna complex / PSII associated light-harvesting complex II / nonphotochemical quenching / sequestering of metal ion / chloroplast stromal thylakoid /  thylakoid lumen / thylakoid lumen /  plastoglobule ...plastid thylakoid membrane / regulation of protein dephosphorylation / plastoglobule ...plastid thylakoid membrane / regulation of protein dephosphorylation /  photoinhibition / photoinhibition /  photosystem II antenna complex / PSII associated light-harvesting complex II / nonphotochemical quenching / sequestering of metal ion / chloroplast stromal thylakoid / photosystem II antenna complex / PSII associated light-harvesting complex II / nonphotochemical quenching / sequestering of metal ion / chloroplast stromal thylakoid /  thylakoid lumen / thylakoid lumen /  plastoglobule / plastoglobule /  thylakoid membrane / chloroplast thylakoid / thylakoid membrane / chloroplast thylakoid /  photosynthesis, light harvesting in photosystem I / chloroplast thylakoid lumen / photosynthesis, light harvesting in photosystem I / chloroplast thylakoid lumen /  apoplast / apoplast /  photosystem II assembly / photosystem II assembly /  photosystem II stabilization / oxygen evolving activity / photosystem II stabilization / oxygen evolving activity /  photosystem II oxygen evolving complex / photosystem II oxygen evolving complex /  photosystem II / photosystem II /  photosystem II reaction center / photosystem II reaction center /  chloroplast envelope / chloroplast envelope /  thylakoid / photosynthetic electron transport chain / oxidoreductase activity, acting on diphenols and related substances as donors, oxygen as acceptor / thylakoid / photosynthetic electron transport chain / oxidoreductase activity, acting on diphenols and related substances as donors, oxygen as acceptor /  photosystem I / poly(U) RNA binding / response to herbicide / photosystem I / poly(U) RNA binding / response to herbicide /  photosystem II / photosystem II /  plastid / plastid /  chloroplast stroma / photosynthetic electron transport in photosystem II / chloroplast stroma / photosynthetic electron transport in photosystem II /  chlorophyll binding / chloroplast thylakoid membrane / chlorophyll binding / chloroplast thylakoid membrane /  photosynthesis, light reaction / electron transporter, transferring electrons within the cyclic electron transport pathway of photosynthesis activity / photosynthesis, light reaction / electron transporter, transferring electrons within the cyclic electron transport pathway of photosynthesis activity /  phosphate ion binding / response to light stimulus / phosphate ion binding / response to light stimulus /  photosynthesis / photosynthesis /  chloroplast / chloroplast /  electron transfer activity / membrane => GO:0016020 / protein stabilization / iron ion binding / protein domain specific binding / electron transfer activity / membrane => GO:0016020 / protein stabilization / iron ion binding / protein domain specific binding /  mRNA binding / mRNA binding /  heme binding / heme binding /  metal ion binding / metal ion binding /  nucleus / nucleus /  plasma membrane / plasma membrane /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   Arabidopsis thaliana (thale cress) Arabidopsis thaliana (thale cress) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 5.3 Å cryo EM / Resolution: 5.3 Å | ||||||
 Authors Authors | van Bezouwen, L.S. / Caffarri, S. / Kale, R.S. / Kouril, R. / Thunnissen, A.M.W.H. / Oostergetel, G.T. / Boekema, E.J. | ||||||
 Citation Citation |  Journal: Nat Plants / Year: 2017 Journal: Nat Plants / Year: 2017Title: Subunit and chlorophyll organization of the plant photosystem II supercomplex. Authors: Laura S van Bezouwen / Stefano Caffarri / Ravindra S Kale / Roman Kouřil / Andy-Mark W H Thunnissen / Gert T Oostergetel / Egbert J Boekema /    Abstract: Photosystem II (PSII) is a light-driven protein, involved in the primary reactions of photosynthesis. In plant photosynthetic membranes PSII forms large multisubunit supercomplexes, containing a ...Photosystem II (PSII) is a light-driven protein, involved in the primary reactions of photosynthesis. In plant photosynthetic membranes PSII forms large multisubunit supercomplexes, containing a dimeric core and up to four light-harvesting complexes (LHCs), which act as antenna proteins. Here we solved a three-dimensional (3D) structure of the CSM supercomplex from Arabidopsis thaliana using cryo-transmission electron microscopy (cryo-EM) and single-particle analysis at an overall resolution of 5.3 Å. Using a combination of homology modelling and restrained refinement against the cryo-EM map, it was possible to model atomic structures for all antenna complexes and almost all core subunits. We located all 35 chlorophylls of the core region based on the cyanobacterial PSII structure, whose positioning is highly conserved, as well as all the chlorophylls of the LHCII S and M trimers. A total of 13 and 9 chlorophylls were identified in CP26 and CP24, respectively. Energy flow from LHC complexes to the PSII reaction centre is proposed to follow preferential pathways: CP26 and CP29 directly transfer to the core using several routes for efficient transfer; the S trimer is directly connected to CP43 and the M trimer can efficiently transfer energy to the core through CP29 and the S trimer. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5mdx.cif.gz 5mdx.cif.gz | 1.7 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5mdx.ent.gz pdb5mdx.ent.gz | 1.5 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5mdx.json.gz 5mdx.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/md/5mdx https://data.pdbj.org/pub/pdb/validation_reports/md/5mdx ftp://data.pdbj.org/pub/pdb/validation_reports/md/5mdx ftp://data.pdbj.org/pub/pdb/validation_reports/md/5mdx | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3491MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Photosystem II ... , 13 types, 26 molecules AaBbCcDdHhIiKkLlMmTtWwXxZz
| #1: Protein |  / PSII D1 protein / Photosystem II Q(B) protein / PSII D1 protein / Photosystem II Q(B) proteinMass: 38003.234 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: P83755, Arabidopsis thaliana (thale cress) / References: UniProt: P83755,  photosystem II photosystem II#2: Protein |  / PSII 47 kDa protein / Protein CP-47 / PSII 47 kDa protein / Protein CP-47Mass: 55960.625 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: P56777 Arabidopsis thaliana (thale cress) / References: UniProt: P56777#3: Protein |  / PSII 43 kDa protein / Protein CP-43 / PSII 43 kDa protein / Protein CP-43Mass: 50086.324 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: P56778 Arabidopsis thaliana (thale cress) / References: UniProt: P56778#4: Protein |  / PSII D2 protein / Photosystem Q(A) protein / PSII D2 protein / Photosystem Q(A) proteinMass: 39444.082 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: P56761, Arabidopsis thaliana (thale cress) / References: UniProt: P56761,  photosystem II photosystem II#7: Protein |  / PSII-H / Photosystem II 10 kDa phosphoprotein / PSII-H / Photosystem II 10 kDa phosphoproteinMass: 7575.691 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: P56780 Arabidopsis thaliana (thale cress) / References: UniProt: P56780#8: Protein/peptide |  / PSII-I / PSII 4.8 kDa protein / PSII-I / PSII 4.8 kDa proteinMass: 4170.891 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: P62100 Arabidopsis thaliana (thale cress) / References: UniProt: P62100#9: Protein/peptide |  / PSII-K / PSII-KMass: 4239.112 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: P56782 Arabidopsis thaliana (thale cress) / References: UniProt: P56782#10: Protein/peptide |  / PSII-L / PSII-LMass: 4471.075 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: P60129 Arabidopsis thaliana (thale cress) / References: UniProt: P60129#11: Protein/peptide |  / PSII-M / PSII-MMass: 3783.538 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: P62109 Arabidopsis thaliana (thale cress) / References: UniProt: P62109#13: Protein/peptide |  / PSII-T / PSII-TMass: 3825.642 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: P61839 Arabidopsis thaliana (thale cress) / References: UniProt: P61839#14: Protein |  / PSII 6.1 kDa protein / PSII 6.1 kDa proteinMass: 6036.749 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: Q39194 Arabidopsis thaliana (thale cress) / References: UniProt: Q39194#15: Protein |  / PSBX / Photosystem II subunit X / Putative PSII-X protein / Putative uncharacterized protein ...PSBX / Photosystem II subunit X / Putative PSII-X protein / Putative uncharacterized protein At2g06520 / T12H3.7 / PSBX / Photosystem II subunit X / Putative PSII-X protein / Putative uncharacterized protein ...PSBX / Photosystem II subunit X / Putative PSII-X protein / Putative uncharacterized protein At2g06520 / T12H3.7Mass: 11826.013 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: Q9SKI3 Arabidopsis thaliana (thale cress) / References: UniProt: Q9SKI3#16: Protein |  / PSII-Z / PSII-ZMass: 6569.768 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: P56790 Arabidopsis thaliana (thale cress) / References: UniProt: P56790 |
|---|
-Cytochrome b559 subunit ... , 2 types, 4 molecules EeFf
| #5: Protein |  / PSII reaction center subunit V / PSII reaction center subunit VMass: 9393.501 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: P56779 Arabidopsis thaliana (thale cress) / References: UniProt: P56779#6: Protein/peptide |  Mass: 4428.228 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: P62095 Arabidopsis thaliana (thale cress) / References: UniProt: P62095 |
|---|
-Protein , 2 types, 4 molecules Oo48
| #12: Protein | Mass: 26594.639 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: P23321 Arabidopsis thaliana (thale cress) / References: UniProt: P23321#20: Protein | Mass: 23014.051 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: Q9LMQ2 Arabidopsis thaliana (thale cress) / References: UniProt: Q9LMQ2 |
|---|
-Chlorophyll a-b binding protein ... , 3 types, 16 molecules RrSsGNYgny123567
| #17: Protein | Mass: 27302.855 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: Q07473 Arabidopsis thaliana (thale cress) / References: UniProt: Q07473#18: Protein | Mass: 25232.754 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: Q9XF89 Arabidopsis thaliana (thale cress) / References: UniProt: Q9XF89#19: Protein | Mass: 23971.910 Da / Num. of mol.: 12 / Source method: isolated from a natural source / Source: (natural)   Arabidopsis thaliana (thale cress) / References: UniProt: P04778 Arabidopsis thaliana (thale cress) / References: UniProt: P04778 |
|---|
-Non-polymers , 5 types, 316 molecules 



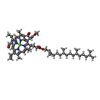




| #21: Chemical | | #22: Chemical | ChemComp-CLA /  Chlorophyll a Chlorophyll a#23: Chemical | ChemComp-PHO /  Pheophytin Pheophytin#24: Chemical |  Heme B Heme B#25: Chemical | ChemComp-CHL /  Chlorophyll b Chlorophyll b |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: C2S2M2 supercomplex of Photosystem II / Type: COMPLEX / Entity ID: #1-#20 / Source: NATURAL / Type: COMPLEX / Entity ID: #1-#20 / Source: NATURAL |
|---|---|
| Source (natural) | Organism:   Arabidopsis thaliana (thale cress) Arabidopsis thaliana (thale cress) |
| Buffer solution | pH: 7.5 |
| Specimen | Conc.: 3.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
| Specimen support | Grid material: COPPER / Grid type: Quantifoil R1.2/1.3 |
Vitrification | Instrument: FEI VITROBOT MARK III / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 293 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 3000 nm / Nominal defocus min: 1200 nm Bright-field microscopy / Nominal defocus max: 3000 nm / Nominal defocus min: 1200 nm |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 1 sec. / Electron dose: 38 e/Å2 / Detector mode: INTEGRATING / Film or detector model: FEI FALCON II (4k x 4k) / Num. of grids imaged: 3 / Num. of real images: 5198 |
| EM imaging optics | Spherical aberration corrector : Microscope had a Cs corrector : Microscope had a Cs corrector |
| Image scans | Movie frames/image: 7 / Used frames/image: 1-7 |
- Processing
Processing
| Software | Name: PHENIX / Version: dev_2474: / Classification: refinement | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| |||||||||||||||||||||||||||||||||||||||||||||
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | |||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry : C2 (2 fold cyclic : C2 (2 fold cyclic ) ) | |||||||||||||||||||||||||||||||||||||||||||||
3D reconstruction | Resolution: 5.3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 23434 / Symmetry type: POINT | |||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: FLEXIBLE FIT / Space: REAL / Target criteria: Cross-correlation coefficient Details: Initial fitting of the subunits in the cryo-EM map was performed by rigid body real space refinement, using as templates the high resolution crystal structures of Thermosynechococcus ...Details: Initial fitting of the subunits in the cryo-EM map was performed by rigid body real space refinement, using as templates the high resolution crystal structures of Thermosynechococcus vulcanus PSII (PDB code 3WU2), pea LHC-II (PDB code 2BHW for the S- and M-trimers, and spinach CP29 (PDB code 3PL9) for CP29, CP26 and CP24. Local fitting and adjustment of the subunits in the cryo-EM maps was performed using manual rebuilding and restrained real space refinement as explained in the primary reference. Due to large differences in local resolution of the cryo-EM map, refinement of the PSII core, the S-trimer with CP26/CP29 and the M-trimer with CP24 was performed separately in excised parts of the cryo-EM map. The core was refined at 4.5 angstrom, the S-trimer+CP26+CP29 at 5.5 angstrom and the M-trimer+CP24 at 6.5 angstrom. Core: chains A,B,C,D,E,F,H,I,J,K,L,M,O,T,W,X,Z and a,b,c,d,e,f,h,i,j,k,l,m,o,t,w,x,z. S-trimer+CP26+CP29: chains G,N,Y,S,R and g,n,y,s,r. M-trimer+CP24: chains 1,2,3,4 and 5,6,7,8. | |||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller







 PDBj
PDBj













