[English] 日本語
 Yorodumi
Yorodumi- EMDB-8125: BG505 SOSIP.664 HIV-1 Env trimer in complex with anti-HIV fusion ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8125 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
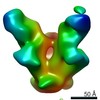
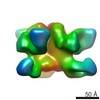
| Title | BG505 SOSIP.664 HIV-1 Env trimer in complex with anti-HIV fusion peptide targeting N123-VRC34.01 Fab | |||||||||
 Map data Map data | None | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / virus-mediated perturbation of host defense response / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell /  viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane /  viral envelope ...positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / virus-mediated perturbation of host defense response / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral envelope ...positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / virus-mediated perturbation of host defense response / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell /  viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane /  viral envelope / structural molecule activity / virion attachment to host cell / host cell plasma membrane / virion membrane / identical protein binding / viral envelope / structural molecule activity / virion attachment to host cell / host cell plasma membrane / virion membrane / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |    Human immunodeficiency virus 1 / Human immunodeficiency virus 1 /   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  negative staining / Resolution: 17.0 Å negative staining / Resolution: 17.0 Å | |||||||||
 Authors Authors | Ozorowski G / Ward AB | |||||||||
 Citation Citation |  Journal: Science / Year: 2016 Journal: Science / Year: 2016Title: Fusion peptide of HIV-1 as a site of vulnerability to neutralizing antibody. Authors: Rui Kong / Kai Xu / Tongqing Zhou / Priyamvada Acharya / Thomas Lemmin / Kevin Liu / Gabriel Ozorowski / Cinque Soto / Justin D Taft / Robert T Bailer / Evan M Cale / Lei Chen / Chang W Choi ...Authors: Rui Kong / Kai Xu / Tongqing Zhou / Priyamvada Acharya / Thomas Lemmin / Kevin Liu / Gabriel Ozorowski / Cinque Soto / Justin D Taft / Robert T Bailer / Evan M Cale / Lei Chen / Chang W Choi / Gwo-Yu Chuang / Nicole A Doria-Rose / Aliaksandr Druz / Ivelin S Georgiev / Jason Gorman / Jinghe Huang / M Gordon Joyce / Mark K Louder / Xiaochu Ma / Krisha McKee / Sijy O'Dell / Marie Pancera / Yongping Yang / Scott C Blanchard / Walther Mothes / Dennis R Burton / Wayne C Koff / Mark Connors / Andrew B Ward / Peter D Kwong / John R Mascola /  Abstract: The HIV-1 fusion peptide, comprising 15 to 20 hydrophobic residues at the N terminus of the Env-gp41 subunit, is a critical component of the virus-cell entry machinery. Here, we report the ...The HIV-1 fusion peptide, comprising 15 to 20 hydrophobic residues at the N terminus of the Env-gp41 subunit, is a critical component of the virus-cell entry machinery. Here, we report the identification of a neutralizing antibody, N123-VRC34.01, which targets the fusion peptide and blocks viral entry by inhibiting conformational changes in gp120 and gp41 subunits of Env required for entry. Crystal structures of N123-VRC34.01 liganded to the fusion peptide, and to the full Env trimer, revealed an epitope consisting of the N-terminal eight residues of the gp41 fusion peptide and glycan N88 of gp120, and molecular dynamics showed that the N-terminal portion of the fusion peptide can be solvent-exposed. These results reveal the fusion peptide to be a neutralizing antibody epitope and thus a target for vaccine design. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8125.map.gz emd_8125.map.gz | 14.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8125-v30.xml emd-8125-v30.xml emd-8125.xml emd-8125.xml | 14.6 KB 14.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8125.png emd_8125.png | 77.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8125 http://ftp.pdbj.org/pub/emdb/structures/EMD-8125 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8125 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8125 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8125.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8125.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.57 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Complex containing 3 copies of N123-VRC34.01 anti-HIV Fab bound t...
| Entire | Name: Complex containing 3 copies of N123-VRC34.01 anti-HIV Fab bound to a trimer of HIV-1 Env B505 SOSIP.664 |
|---|---|
| Components |
|
-Supramolecule #1: Complex containing 3 copies of N123-VRC34.01 anti-HIV Fab bound t...
| Supramolecule | Name: Complex containing 3 copies of N123-VRC34.01 anti-HIV Fab bound to a trimer of HIV-1 Env B505 SOSIP.664 type: complex / ID: 1 / Parent: 0 |
|---|---|
| Molecular weight | Theoretical: 570 KDa |
-Supramolecule #2: HIV-1 Env BG505 SOSIP.664
| Supramolecule | Name: HIV-1 Env BG505 SOSIP.664 / type: complex / ID: 2 / Parent: 1 Details: Soluble and stabilized HIV-1 Env trimer from strain BG505. Engineered disulfide between A501C and T605C. I559P mutation to stabilize in pre-fusion state. Addition of N332 to restore ...Details: Soluble and stabilized HIV-1 Env trimer from strain BG505. Engineered disulfide between A501C and T605C. I559P mutation to stabilize in pre-fusion state. Addition of N332 to restore glycosylation site for purification and antigenic properties. Truncation after D664 to increase solubility. Formed with three gp140 subunits. |
|---|---|
| Source (natural) | Organism:    Human immunodeficiency virus 1 / Strain: BG505 Human immunodeficiency virus 1 / Strain: BG505 |
| Recombinant expression | Organism:   Homo sapiens (human) / Recombinant cell: HEK293F / Recombinant plasmid: pPPI4 Homo sapiens (human) / Recombinant cell: HEK293F / Recombinant plasmid: pPPI4 |
-Supramolecule #3: Anti-HIV N123-VRC34.01 antibody fragment antigen binding
| Supramolecule | Name: Anti-HIV N123-VRC34.01 antibody fragment antigen binding type: complex / ID: 3 / Parent: 1 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:   Homo sapiens (human) / Recombinant cell: Expi 293 / Recombinant plasmid: pcDNA3.1 Homo sapiens (human) / Recombinant cell: Expi 293 / Recombinant plasmid: pcDNA3.1 |
| Molecular weight | Theoretical: 50 KDa |
-Experimental details
-Structure determination
| Method |  negative staining negative staining |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.03 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: Sterile filtered buffer | |||||||||
| Staining | Type: NEGATIVE / Material: 2% w/v uranyl formate Details: Negatively stained EM samples were prepared on carbon-coated Cu400 grids by applying sample for 10 seconds, blotting, applying 2% w/v uranyl formate for 45 seconds, and blotting again. | |||||||||
| Grid | Model: EMS / Material: COPPER / Support film - Material: CARBON / Pretreatment - Type: PLASMA CLEANING | |||||||||
| Details | Trimers were incubated with a 6-molar excess of Fab overnight at room temperature. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI 20 |
|---|---|
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 92000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 92000 |
| Sample stage | Specimen holder model: SIDE ENTRY, EUCENTRIC |
| Details | Collected a tilt series of -50, -40, -30, -20, -10, and 0 degrees. |
| Image recording | Film or detector model: FEI CETA (4k x 4k) / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Digitization - Sampling interval: 14.0 µm / Average electron dose: 25.0 e/Å2 |
- Image processing
Image processing
| Startup model | Type of model: NONE Details: EMAN2 e2initialmodel.py was used to generate an initial model from 2D class averages. |
|---|---|
| Initial angle assignment | Type: PROJECTION MATCHING / Software - Name: EMAN2 / Software - details: e2initialmodel.py |
| Final angle assignment | Type: PROJECTION MATCHING / Software - Name: SPARX / Software - details: sxali3d.py Details: Sparx sxali3d.py was used to refine particles against an initial model generated by EMAN2. |
| Final reconstruction | Applied symmetry - Point group: C3 (3 fold cyclic ) / Resolution.type: BY AUTHOR / Resolution: 17.0 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: SPARX / Software - details: sxali3d.py / Number images used: 9261 ) / Resolution.type: BY AUTHOR / Resolution: 17.0 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: SPARX / Software - details: sxali3d.py / Number images used: 9261 |
| Details | Dark and light camera corrections were performed prior to data acquisition. |
 Movie
Movie Controller
Controller