+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-7075 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Xenopus Tropicalis TRPV4 | ||||||||||||
 Map data Map data | Transient receptor potential cation channel, subfamily V, member 4 | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords |  Ion Channel / Ion Channel /  TRP channel / METAL TRANSPORT TRP channel / METAL TRANSPORT | ||||||||||||
| Function / homology |  Function and homology information Function and homology information TRP channels / osmosensory signaling pathway / plasma membrane => GO:0005886 / calcium ion import across plasma membrane / actin filament organization / TRP channels / osmosensory signaling pathway / plasma membrane => GO:0005886 / calcium ion import across plasma membrane / actin filament organization /  calcium channel activity / calcium channel activity /  cilium / monoatomic ion channel activity cilium / monoatomic ion channel activitySimilarity search - Function | ||||||||||||
| Biological species |  Xenopus tropicalis (tropical clawed frog) Xenopus tropicalis (tropical clawed frog) | ||||||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.8 Å cryo EM / Resolution: 3.8 Å | ||||||||||||
 Authors Authors | Deng Z / Hite RK | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2018 Journal: Nat Struct Mol Biol / Year: 2018Title: Cryo-EM and X-ray structures of TRPV4 reveal insight into ion permeation and gating mechanisms. Authors: Zengqin Deng / Navid Paknejad / Grigory Maksaev / Monica Sala-Rabanal / Colin G Nichols / Richard K Hite / Peng Yuan /  Abstract: The transient receptor potential (TRP) channel TRPV4 participates in multiple biological processes, and numerous TRPV4 mutations underlie several distinct and devastating diseases. Here we present ...The transient receptor potential (TRP) channel TRPV4 participates in multiple biological processes, and numerous TRPV4 mutations underlie several distinct and devastating diseases. Here we present the cryo-EM structure of Xenopus tropicalis TRPV4 at 3.8-Å resolution. The ion-conduction pore contains an intracellular gate formed by the inner helices, but lacks any extracellular gate in the selectivity filter, as observed in other TRPV channels. Anomalous X-ray diffraction analyses identify a single ion-binding site in the selectivity filter, thus explaining TRPV4 nonselectivity. Structural comparisons with other TRP channels and distantly related voltage-gated cation channels reveal an unprecedented, unique packing interface between the voltage-sensor-like domain and the pore domain, suggesting distinct gating mechanisms. Moreover, our structure begins to provide mechanistic insights to the large set of pathogenic mutations, offering potential opportunities for drug development. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_7075.map.gz emd_7075.map.gz | 1.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-7075-v30.xml emd-7075-v30.xml emd-7075.xml emd-7075.xml | 20.2 KB 20.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_7075.png emd_7075.png | 174.9 KB | ||
| Filedesc metadata |  emd-7075.cif.gz emd-7075.cif.gz | 6.6 KB | ||
| Others |  emd_7075_half_map_1.map.gz emd_7075_half_map_1.map.gz emd_7075_half_map_2.map.gz emd_7075_half_map_2.map.gz | 53.8 MB 53.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-7075 http://ftp.pdbj.org/pub/emdb/structures/EMD-7075 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7075 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7075 | HTTPS FTP |
-Related structure data
| Related structure data |  6bbjMC  6c8fC  6c8gC  6c8hC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_7075.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_7075.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Transient receptor potential cation channel, subfamily V, member 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.088 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
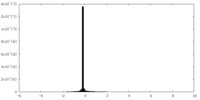
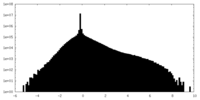
-Half map: Transient receptor potential cation channel, subfamily V, member 4
| File | emd_7075_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Transient receptor potential cation channel, subfamily V, member 4 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
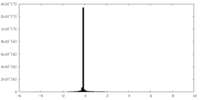
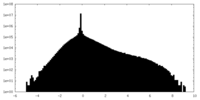
-Half map: Transient receptor potential cation channel, subfamily V, member 4
| File | emd_7075_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Transient receptor potential cation channel, subfamily V, member 4 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : TRPV4
| Entire | Name: TRPV4 |
|---|---|
| Components |
|
-Supramolecule #1: TRPV4
| Supramolecule | Name: TRPV4 / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all Details: Xenopis tropicalis TRPV4 purified in DDM and lipids |
|---|---|
| Source (natural) | Organism:  Xenopus tropicalis (tropical clawed frog) Xenopus tropicalis (tropical clawed frog) |
| Molecular weight | Theoretical: 300 KDa |
-Macromolecule #1: Transient receptor potential cation channel, subfamily V, member 4
| Macromolecule | Name: Transient receptor potential cation channel, subfamily V, member 4 type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Xenopus tropicalis (tropical clawed frog) Xenopus tropicalis (tropical clawed frog) |
| Molecular weight | Theoretical: 99.087852 KDa |
| Recombinant expression | Organism:   Pichia (fungus) Pichia (fungus) |
| Sequence | String: MADPSHLLKH NASVDIDDSQ GDDGSNHNDS FPLSSLANLF ENEESSAPNE GVRSPQVPGD NKQNLRIRFQ GPFRKGISNP MDLLESTIY ESSAPKKAPM DSLFGYETYH HHPTENRRKR KKILLEKENL NSQAPSPDPP PVIKMFNRHM LFDIVSRGST A ELEGFLPF ...String: MADPSHLLKH NASVDIDDSQ GDDGSNHNDS FPLSSLANLF ENEESSAPNE GVRSPQVPGD NKQNLRIRFQ GPFRKGISNP MDLLESTIY ESSAPKKAPM DSLFGYETYH HHPTENRRKR KKILLEKENL NSQAPSPDPP PVIKMFNRHM LFDIVSRGST A ELEGFLPF LLAQKKRLTD EEFREASTGK TCLTKALMNL NGGKNDTIPM LIDIAEKTGN LREFINSPFR DVYYRGQTAL HI AIERRCK HYVELLVEKG ADVHAQARGR FFQPKDEGGY FYFGELPLSL AACTNQPDIV HYLTENAHKK ADIRRQDSRG NTV LHALVA IADNTRENTK FVTKVYDLLV IKCVKLYPDS SLEAIFNNDS MSPLMMAAKL GKIGIFQHII RLEIKDEEAR HLSR KFRDW AYGPVYSSLY DLSMLDTCGE EVSVLEILVY NSKVENRHEM LAVEPINELL RDKWQKFGAV SFYISVVSYL IAMII FTLI AYYRPMDGTP PYPYRTTMDY MRLAGEIVTL LTGVVFFITN IKDLFMKKCP GVNSLFIDGS FQLLYFIYSV LVIITA VLY LVGIESYLAV MVFALVLGWM NALYFTRGLK LTGTYSIMLQ KILFKDLFRF LLVYLLFMIG YASALVSLLN PCTSQES CI ETSSNCTVPE YPSCRDSSTF SKFLLDLFKL TIGMGDLEMI NSAKYPAVFI ILLVTYIILT FVLLLNMLIA LMGETVGQ V SKESKQIWKL QWATTILDIE RSFPVCMRKA FRSGEMVTVG KNLDGTPDRR WCFRVDEVNW SHWNQNLGII NEDPGRNDG YQYYGFSQTV GRLRRDRWSV VVPRVVELNK APQHSDDVVV PLGNIPQVQT YSQRQENAQN WKKDETHI UniProtKB: Transient receptor potential cation channel, subfamily V, member 4 |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4.5 mg/mL | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 10 sec. / Pretreatment - Atmosphere: AIR | ||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 298 K / Instrument: FEI VITROBOT MARK IV / Details: 2 second blot time. | ||||||||||||||||||||||||
| Details | TRPV4 in DDM and lipids |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated defocus max: 2.5 µm / Calibrated defocus min: 0.8 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 22500 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 22500 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Dimensions - Width: 7420 pixel / Digitization - Dimensions - Height: 7676 pixel / Digitization - Frames/image: 2-20 / Number grids imaged: 1 / Number real images: 1604 / Average exposure time: 4.0 sec. / Average electron dose: 27.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Particle selection | Number selected: 85253 |
|---|---|
| Startup model | Type of model: OTHER / Details: Initial model generated in cryosparc |
| Initial angle assignment | Type: NOT APPLICABLE |
| Final 3D classification | Number classes: 5 / Avg.num./class: 17000 / Software - Name: RELION (ver. 2.1) |
| Final angle assignment | Type: NOT APPLICABLE |
| Final reconstruction | Number classes used: 4 / Applied symmetry - Point group: C4 (4 fold cyclic ) / Resolution.type: BY AUTHOR / Resolution: 3.8 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: FREALIGN (ver. 9.11) ) / Resolution.type: BY AUTHOR / Resolution: 3.8 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: FREALIGN (ver. 9.11)Details: Refined using Frealign with reference map low-pass filtered to 6 Angstroms Number images used: 72684 |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Target criteria: FSC |
|---|---|
| Output model |  PDB-6bbj: |
 Movie
Movie Controller
Controller















 Z
Z Y
Y X
X