+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5917 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Encapsulin protein (EncA) from Myxococcus xanthus | |||||||||
 Map data Map data | Reconstruction of the encapsulin protein (EncA) from Myxococcus xanthus | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  encapsulin / encapsulin /  Myxococcus xanthus / HK-97 fold / protein-bound organelle / iron storage Myxococcus xanthus / HK-97 fold / protein-bound organelle / iron storage | |||||||||
| Function / homology | Type 1 encapsulin shell protein / Type 1 encapsulin shell protein / Encapsulating protein for peroxidase /  encapsulin nanocompartment / encapsulin nanocompartment /  peptidase activity / iron ion transport / intracellular iron ion homeostasis / defense response to bacterium / Type 1 encapsulin shell protein EncA peptidase activity / iron ion transport / intracellular iron ion homeostasis / defense response to bacterium / Type 1 encapsulin shell protein EncA Function and homology information Function and homology information | |||||||||
| Biological species |   Myxococcus xanthus (bacteria) Myxococcus xanthus (bacteria) | |||||||||
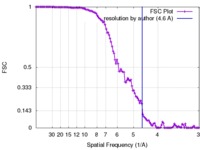
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 4.6 Å cryo EM / Resolution: 4.6 Å | |||||||||
 Authors Authors | McHugh CA / Fontana J / Lam AS / Cheng N / Aksyuk AA / Steven AC / Hoiczyk E | |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2014 Journal: EMBO J / Year: 2014Title: A virus capsid-like nanocompartment that stores iron and protects bacteria from oxidative stress. Authors: Colleen A McHugh / Juan Fontana / Daniel Nemecek / Naiqian Cheng / Anastasia A Aksyuk / J Bernard Heymann / Dennis C Winkler / Alan S Lam / Joseph S Wall / Alasdair C Steven / Egbert Hoiczyk /  Abstract: Living cells compartmentalize materials and enzymatic reactions to increase metabolic efficiency. While eukaryotes use membrane-bound organelles, bacteria and archaea rely primarily on protein-bound ...Living cells compartmentalize materials and enzymatic reactions to increase metabolic efficiency. While eukaryotes use membrane-bound organelles, bacteria and archaea rely primarily on protein-bound nanocompartments. Encapsulins constitute a class of nanocompartments widespread in bacteria and archaea whose functions have hitherto been unclear. Here, we characterize the encapsulin nanocompartment from Myxococcus xanthus, which consists of a shell protein (EncA, 32.5 kDa) and three internal proteins (EncB, 17 kDa; EncC, 13 kDa; EncD, 11 kDa). Using cryo-electron microscopy, we determined that EncA self-assembles into an icosahedral shell 32 nm in diameter (26 nm internal diameter), built from 180 subunits with the fold first observed in bacteriophage HK97 capsid. The internal proteins, of which EncB and EncC have ferritin-like domains, attach to its inner surface. Native nanocompartments have dense iron-rich cores. Functionally, they resemble ferritins, cage-like iron storage proteins, but with a massively greater capacity (~30,000 iron atoms versus ~3,000 in ferritin). Physiological data reveal that few nanocompartments are assembled during vegetative growth, but they increase fivefold upon starvation, protecting cells from oxidative stress through iron sequestration. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5917.map.gz emd_5917.map.gz | 317.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5917-v30.xml emd-5917-v30.xml emd-5917.xml emd-5917.xml | 10.7 KB 10.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_5917_fsc.xml emd_5917_fsc.xml | 11.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_5917.png emd_5917.png | 291.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5917 http://ftp.pdbj.org/pub/emdb/structures/EMD-5917 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5917 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5917 | HTTPS FTP |
-Related structure data
| Related structure data |  4pt2MC  5953C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_5917.map.gz / Format: CCP4 / Size: 335 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5917.map.gz / Format: CCP4 / Size: 335 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of the encapsulin protein (EncA) from Myxococcus xanthus | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.102 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Encapsulin protein (EncA) from Myxococcus xanthus
| Entire | Name: Encapsulin protein (EncA) from Myxococcus xanthus |
|---|---|
| Components |
|
-Supramolecule #1000: Encapsulin protein (EncA) from Myxococcus xanthus
| Supramolecule | Name: Encapsulin protein (EncA) from Myxococcus xanthus / type: sample / ID: 1000 / Oligomeric state: Icosahedral / Number unique components: 1 |
|---|---|
| Molecular weight | Theoretical: 32 KDa |
-Macromolecule #1: Encapsulin
| Macromolecule | Name: Encapsulin / type: protein_or_peptide / ID: 1 / Name.synonym: EncA / Number of copies: 1 / Oligomeric state: Icosahedral / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:   Myxococcus xanthus (bacteria) / Strain: DK 1622 Myxococcus xanthus (bacteria) / Strain: DK 1622 |
| Molecular weight | Theoretical: 32 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) / Recombinant strain: BL21 / Recombinant plasmid: pET12a-3556 Escherichia coli (E. coli) / Recombinant strain: BL21 / Recombinant plasmid: pET12a-3556 |
| Sequence | UniProtKB: Type 1 encapsulin shell protein EncA / GO: defense response to bacterium,  peptidase activity / InterPro: Type 1 encapsulin shell protein peptidase activity / InterPro: Type 1 encapsulin shell protein |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL |
|---|---|
| Buffer | pH: 7.6 / Details: 10 mM Tris, 10 mM MgCl2 |
| Grid | Details: 400 mesh carbon grid with thin carbon support |
| Vitrification | Cryogen name: ETHANE / Chamber temperature: 83 K / Instrument: LEICA KF80 |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 57620 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 1.65 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 59000 Bright-field microscopy / Nominal defocus max: 1.65 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 59000 |
| Sample stage | Specimen holder model: OTHER |
| Date | Dec 3, 2012 |
| Image recording | Digitization - Scanner: NIKON SUPER COOLSCAN 9000 / Digitization - Sampling interval: 6.35 µm / Number real images: 157 / Average electron dose: 15 e/Å2 |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller