+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3821 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM reconstruction of a modified human Adenovirus C5 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationT=25 icosahedral viral capsid / microtubule-dependent intracellular transport of viral material towards nucleus /  viral capsid / symbiont entry into host cell / host cell nucleus / structural molecule activity viral capsid / symbiont entry into host cell / host cell nucleus / structural molecule activitySimilarity search - Function | |||||||||
| Biological species |    Human adenovirus 5 Human adenovirus 5 | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 7.4 Å cryo EM / Resolution: 7.4 Å | |||||||||
 Authors Authors | Schmid M / Ernst P / Honegger A / Suomalainen M / Zimmermann M / Braun L / Stauffer S / Thom C / Dreier B / Eibauer M ...Schmid M / Ernst P / Honegger A / Suomalainen M / Zimmermann M / Braun L / Stauffer S / Thom C / Dreier B / Eibauer M / Kipar A / Vogel V / Greber UF / Medalia O / Plueckthun A | |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2018 Journal: Nat Commun / Year: 2018Title: Adenoviral vector with shield and adapter increases tumor specificity and escapes liver and immune control. Authors: Markus Schmid / Patrick Ernst / Annemarie Honegger / Maarit Suomalainen / Martina Zimmermann / Lukas Braun / Sarah Stauffer / Cristian Thom / Birgit Dreier / Matthias Eibauer / Anja Kipar / ...Authors: Markus Schmid / Patrick Ernst / Annemarie Honegger / Maarit Suomalainen / Martina Zimmermann / Lukas Braun / Sarah Stauffer / Cristian Thom / Birgit Dreier / Matthias Eibauer / Anja Kipar / Viola Vogel / Urs F Greber / Ohad Medalia / Andreas Plückthun /   Abstract: Most systemic viral gene therapies have been limited by sequestration and degradation of virions, innate and adaptive immunity, and silencing of therapeutic genes within the target cells. Here we ...Most systemic viral gene therapies have been limited by sequestration and degradation of virions, innate and adaptive immunity, and silencing of therapeutic genes within the target cells. Here we engineer a high-affinity protein coat, shielding the most commonly used vector in clinical gene therapy, human adenovirus type 5. Using electron microscopy and crystallography we demonstrate a massive coverage of the virion surface through the hexon-shielding scFv fragment, trimerized to exploit the hexon symmetry and gain avidity. The shield reduces virion clearance in the liver. When the shielded particles are equipped with adaptor proteins, the virions deliver their payload genes into human cancer cells expressing HER2 or EGFR. The combination of shield and adapter also increases viral gene delivery to xenografted tumors in vivo, reduces liver off-targeting and immune neutralization. Our study highlights the power of protein engineering for viral vectors overcoming the challenges of local and systemic viral gene therapies. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3821.map.gz emd_3821.map.gz | 283.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3821-v30.xml emd-3821-v30.xml emd-3821.xml emd-3821.xml | 13.5 KB 13.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_3821_fsc.xml emd_3821_fsc.xml | 28.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_3821.png emd_3821.png | 211.9 KB | ||
| Others |  emd_3821_half_map_1.map.gz emd_3821_half_map_1.map.gz emd_3821_half_map_2.map.gz emd_3821_half_map_2.map.gz | 1.5 GB 1.5 GB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3821 http://ftp.pdbj.org/pub/emdb/structures/EMD-3821 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3821 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3821 | HTTPS FTP |
-Related structure data
| Related structure data |  6eqcMC  5ogiC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10117 (Title: Cryo-EM reconstruction of a modified human Adenovirus C5 EMPIAR-10117 (Title: Cryo-EM reconstruction of a modified human Adenovirus C5Data size: 612.7 Data #1: Unaligned multi-frame micrographs of a modified human Adenovirus C5 [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_3821.map.gz / Format: CCP4 / Size: 1.9 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3821.map.gz / Format: CCP4 / Size: 1.9 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.96 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: #1
| File | emd_3821_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_3821_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human adenovirus 5
| Entire | Name:    Human adenovirus 5 Human adenovirus 5 |
|---|---|
| Components |
|
-Supramolecule #1: Human adenovirus 5
| Supramolecule | Name: Human adenovirus 5 / type: virus / ID: 1 / Parent: 0 / NCBI-ID: 28285 / Sci species name: Human adenovirus 5 / Virus type: VIRION / Virus isolate: SEROTYPE / Virus enveloped: No / Virus empty: No |
|---|
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy Bright-field microscopy |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average exposure time: 0.4 sec. / Average electron dose: 1.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| CTF correction | Software - Name: CTFFIND (ver. 4.0.17) |
|---|---|
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID:  4cwu |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 7.4 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 1.4) / Number images used: 1880 |
FSC plot (resolution estimation) | 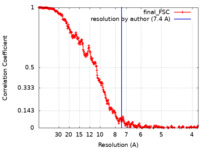 |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-6eqc: |
 Movie
Movie Controller
Controller












 Z
Z Y
Y X
X