[English] 日本語
 Yorodumi
Yorodumi- EMDB-1699: Structure of Lactococcal Phage p2 Baseplate and its Mechanism of ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1699 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of Lactococcal Phage p2 Baseplate and its Mechanism of Activation | |||||||||
 Map data Map data | This is the ccp4 file of the EM 3D reconstruction of the baseplate of the wild-type p2 bacteriophage. The map is associated to the following PDB entries: PDB: 2WZP: BP closed form PDB: 2X53: BP Activated form C2 PDB: 2X54 + 2X5A: BP Activated form P2 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | p2 /  baseplate / baseplate /  phage / phage /  EM EM | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont genome ejection through host cell envelope, long flexible tail mechanism / virus tail, baseplate / virus tail / entry receptor-mediated virion attachment to host cell /  cell adhesion / symbiont entry into host cell / virion attachment to host cell cell adhesion / symbiont entry into host cell / virion attachment to host cellSimilarity search - Function | |||||||||
| Biological species |  Lactococcus phage p2 (virus) Lactococcus phage p2 (virus) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  negative staining / Resolution: 22.0 Å negative staining / Resolution: 22.0 Å | |||||||||
 Authors Authors | Sciara G / Bebeacua C / Bron P / Tremblay D / Ortiz-Lombardia M / Lichiere J / van Heel M / Campanacci V / Moineau S / Cambillau C | |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2010 Journal: Proc Natl Acad Sci U S A / Year: 2010Title: Structure of lactococcal phage p2 baseplate and its mechanism of activation. Authors: Giuliano Sciara / Cecilia Bebeacua / Patrick Bron / Denise Tremblay / Miguel Ortiz-Lombardia / Julie Lichière / Marin van Heel / Valérie Campanacci / Sylvain Moineau / Christian Cambillau /  Abstract: Siphoviridae is the most abundant viral family on earth which infects bacteria as well as archaea. All known siphophages infecting gram+ Lactococcus lactis possess a baseplate at the tip of their ...Siphoviridae is the most abundant viral family on earth which infects bacteria as well as archaea. All known siphophages infecting gram+ Lactococcus lactis possess a baseplate at the tip of their tail involved in host recognition and attachment. Here, we report analysis of the p2 phage baseplate structure by X-ray crystallography and electron microscopy and propose a mechanism for the baseplate activation during attachment to the host cell. This approximately 1 MDa, Escherichia coli-expressed baseplate is composed of three protein species, including six trimers of the receptor-binding protein (RBP). RBPs host-recognition domains point upwards, towards the capsid, in agreement with the electron-microscopy map of the free virion. In the presence of Ca(2+), a cation mandatory for infection, the RBPs rotated 200 degrees downwards, presenting their binding sites to the host, and a channel opens at the bottom of the baseplate for DNA passage. These conformational changes reveal a novel siphophage activation and host-recognition mechanism leading ultimately to DNA ejection. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1699.map.gz emd_1699.map.gz | 828.7 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1699-v30.xml emd-1699-v30.xml emd-1699.xml emd-1699.xml | 9 KB 9 KB | Display Display |  EMDB header EMDB header |
| Images |  3d_p2baseplate.png 3d_p2baseplate.png | 80.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1699 http://ftp.pdbj.org/pub/emdb/structures/EMD-1699 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1699 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1699 | HTTPS FTP |
-Related structure data
| Related structure data |  6zjjM 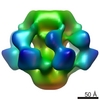 1706C  2wzpC  2x53C  4v5iC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_1699.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1699.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | This is the ccp4 file of the EM 3D reconstruction of the baseplate of the wild-type p2 bacteriophage. The map is associated to the following PDB entries: PDB: 2WZP: BP closed form PDB: 2X53: BP Activated form C2 PDB: 2X54 + 2X5A: BP Activated form P2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.64 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : P2 baseplate wild-type
| Entire | Name: P2 baseplate wild-type |
|---|---|
| Components |
|
-Supramolecule #1000: P2 baseplate wild-type
| Supramolecule | Name: P2 baseplate wild-type / type: sample / ID: 1000 / Oligomeric state: Homohexamer / Number unique components: 1 |
|---|---|
| Molecular weight | Experimental: 1 MDa / Theoretical: 1 MDa |
-Macromolecule #1: P2 baseplate
| Macromolecule | Name: P2 baseplate / type: protein_or_peptide / ID: 1 / Name.synonym: P2 baseplate / Number of copies: 6 / Oligomeric state: Hexamer / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:  Lactococcus phage p2 (virus) Lactococcus phage p2 (virus) |
-Experimental details
-Structure determination
| Method |  negative staining negative staining |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Staining | Type: NEGATIVE Details: Sample was incubated on glow-discharged grid for approximately one minute. 2% uranyl acetate was applied onto the sample and left for about 30 seconds. |
|---|---|
| Vitrification | Cryogen name: NONE / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI/PHILIPS CM200FEG/UT |
|---|---|
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.2 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 38000 Bright-field microscopy / Cs: 2.2 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 38000 |
| Sample stage | Specimen holder: Room Temperature / Specimen holder model: OTHER |
| Alignment procedure | Legacy - Astigmatism: corrected at 200,000 times magnification |
| Image recording | Digitization - Sampling interval: 2.32 µm / Number real images: 1000 / Average electron dose: 10 e/Å2 |
| Tilt angle min | 0 |
| Tilt angle max | 0 |
- Image processing
Image processing
| CTF correction | Details: CCD Images |
|---|---|
| Final angle assignment | Details: IMAGIC |
| Final reconstruction | Applied symmetry - Point group: C6 (6 fold cyclic ) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 22.0 Å / Resolution method: OTHER / Software - Name: IMAGIC-5 ) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 22.0 Å / Resolution method: OTHER / Software - Name: IMAGIC-5Details: Initial map calculated with class averages. Final map calculated after projection matching refinement. Number images used: 9486 |
 Movie
Movie Controller
Controller










