+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8714 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM reconstruction of B41 SOSIP.664 | |||||||||
 Map data Map data | Cryo-EM reconstruction of B41 SOSIP.664 | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / virus-mediated perturbation of host defense response / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell /  viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane /  viral envelope ...positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / virus-mediated perturbation of host defense response / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral envelope ...positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / virus-mediated perturbation of host defense response / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell /  viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane /  viral envelope / structural molecule activity / virion attachment to host cell / host cell plasma membrane / virion membrane / viral envelope / structural molecule activity / virion attachment to host cell / host cell plasma membrane / virion membrane /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |    Human immunodeficiency virus 1 Human immunodeficiency virus 1 | |||||||||
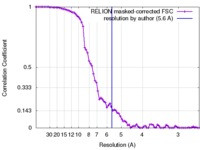
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 5.6 Å cryo EM / Resolution: 5.6 Å | |||||||||
 Authors Authors | Pallesen J / Ozorowski G / de Val N / Ward AB | |||||||||
 Citation Citation |  Journal: Nature / Year: 2017 Journal: Nature / Year: 2017Title: Open and closed structures reveal allostery and pliability in the HIV-1 envelope spike. Authors: Gabriel Ozorowski / Jesper Pallesen / Natalia de Val / Dmitry Lyumkis / Christopher A Cottrell / Jonathan L Torres / Jeffrey Copps / Robyn L Stanfield / Albert Cupo / Pavel Pugach / John P ...Authors: Gabriel Ozorowski / Jesper Pallesen / Natalia de Val / Dmitry Lyumkis / Christopher A Cottrell / Jonathan L Torres / Jeffrey Copps / Robyn L Stanfield / Albert Cupo / Pavel Pugach / John P Moore / Ian A Wilson / Andrew B Ward /  Abstract: For many enveloped viruses, binding to a receptor(s) on a host cell acts as the first step in a series of events culminating in fusion with the host cell membrane and transfer of genetic material for ...For many enveloped viruses, binding to a receptor(s) on a host cell acts as the first step in a series of events culminating in fusion with the host cell membrane and transfer of genetic material for replication. The envelope glycoprotein (Env) trimer on the surface of HIV is responsible for receptor binding and fusion. Although Env can tolerate a high degree of mutation in five variable regions (V1-V5), and also at N-linked glycosylation sites that contribute roughly half the mass of Env, the functional sites for recognition of receptor CD4 and co-receptor CXCR4/CCR5 are conserved and essential for viral fitness. Soluble SOSIP Env trimers are structural and antigenic mimics of the pre-fusion native, surface-presented Env, and are targets of broadly neutralizing antibodies. Thus, they are attractive immunogens for vaccine development. Here we present high-resolution cryo-electron microscopy structures of subtype B B41 SOSIP Env trimers in complex with CD4 and antibody 17b, or with antibody b12, at resolutions of 3.7 Å and 3.6 Å, respectively. We compare these to cryo-electron microscopy reconstructions of B41 SOSIP Env trimers with no ligand or in complex with either CD4 or the CD4-binding-site antibody PGV04 at 5.6 Å, 5.2 Å and 7.4 Å resolution, respectively. Consequently, we present the most complete description yet, to our knowledge, of the CD4-17b-induced intermediate and provide the molecular basis of the receptor-binding-induced conformational change required for HIV-1 entry into host cells. Both CD4 and b12 induce large, previously uncharacterized conformational rearrangements in the gp41 subunits, and the fusion peptide becomes buried in a newly formed pocket. These structures provide key details on the biological function of the type I viral fusion machine from HIV-1 as well as new templates for inhibitor design. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8714.map.gz emd_8714.map.gz | 37.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8714-v30.xml emd-8714-v30.xml emd-8714.xml emd-8714.xml | 23 KB 23 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_8714_fsc.xml emd_8714_fsc.xml | 7.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_8714.png emd_8714.png | 77.7 KB | ||
| Others |  emd_8714_additional.map.gz emd_8714_additional.map.gz emd_8714_half_map_1.map.gz emd_8714_half_map_1.map.gz emd_8714_half_map_2.map.gz emd_8714_half_map_2.map.gz | 36.4 MB 31.4 MB 31.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8714 http://ftp.pdbj.org/pub/emdb/structures/EMD-8714 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8714 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8714 | HTTPS FTP |
-Related structure data
| Related structure data |  8713C  8715C  8716C  8717C  8729C  8730C  5vn3C  5vn8C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8714.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8714.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM reconstruction of B41 SOSIP.664 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.31 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Cryo-EM reconstruction of B41 SOSIP.664, additional map
| File | emd_8714_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM reconstruction of B41 SOSIP.664, additional map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_8714_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_8714_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : HIV-1 Env B41 SOSIP.664
| Entire | Name: HIV-1 Env B41 SOSIP.664 |
|---|---|
| Components |
|
-Supramolecule #1: HIV-1 Env B41 SOSIP.664
| Supramolecule | Name: HIV-1 Env B41 SOSIP.664 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:    Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Recombinant expression | Organism:   homo sapiens (human) / Recombinant cell: HEK293F homo sapiens (human) / Recombinant cell: HEK293F |
| Molecular weight | Theoretical: 480 KDa |
-Macromolecule #1: HIV-1 Env B41 SOSIP.664 gp41
| Macromolecule | Name: HIV-1 Env B41 SOSIP.664 gp41 / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:    Human immunodeficiency virus 1 / Strain: 9032-08.A1.4685 Human immunodeficiency virus 1 / Strain: 9032-08.A1.4685 |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: AVGLGA FIL GFLGAAG ST MGAASMAL T VQARLLLSG IVQQQNNLLR APEAQQHML Q LTVWGIKQ LQ ARVLAVE RYL RDQQLL GIWG CSGKI ICCTN VPWN DSWSNK TIN EIWDNMT WM QWEKEIDN Y TQHIYTLLE VSQIQQEKNE QELLELD |
-Macromolecule #2: HIV-1 Env B41 SOSIP.664 gp120
| Macromolecule | Name: HIV-1 Env B41 SOSIP.664 gp120 / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:    Human immunodeficiency virus 1 / Strain: 9032-08.A1.4685 Human immunodeficiency virus 1 / Strain: 9032-08.A1.4685 |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDAMKRGLCC VLLLCGAVF V SPSQEIHA RF RRGARAA KKW VTVYYG VPVW KEATT TLFCA SDAK AYDTEV HNV WATHACV PT DPNPQEIV L GNVTENFNM WKNNMVEQMH EDIISLWDQ S LKPCVKLT PL CVTLNCN NVN TNNTNN STNA TISDW ...String: MDAMKRGLCC VLLLCGAVF V SPSQEIHA RF RRGARAA KKW VTVYYG VPVW KEATT TLFCA SDAK AYDTEV HNV WATHACV PT DPNPQEIV L GNVTENFNM WKNNMVEQMH EDIISLWDQ S LKPCVKLT PL CVTLNCN NVN TNNTNN STNA TISDW EKMET GEMK NCSFNV TTS IRDKIKK EY ALFYKLDV V PLENKNNIN NTNITNYRLI NCNTSVITQ A CPKVSFEP IP IHYCAPA GFA ILKCNS KTFN GSGPC TNVST VQCT HGIRPV VST QLLLNGS LA EEEIVIRS E NITDNAKTI IVQLNEAVEI NCTRPNNNT R KSIHIGPG RA FYATGDI IGN IRQAHC NISK ARWNE TLGQI VAKL EEQFPN KTI IFNHSSG GD PEIVTHSF N CGGEFFYCN TTPLFNSTWN NTRTDDYPT G GEQNITLQ CR IKQIINM WQG VGKAMY APPI RGQIR CSSNI TGLL LTRDGG RDQ NGTETFR PG GGNMRDNW R SELYKYKVV KIEPLGIAPT ACKRRVVQR RRR |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: DDM was added to a final concentration of 0.06 mM prior to vitrification | ||||||||||||
| Grid | Model: C-flat-2/2 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Atmosphere: OTHER | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber temperature: 277 K / Instrument: HOMEMADE PLUNGER Details: 3 uL of sample applied to a holey carbon grid on glow discharged face and blotted manually on sample side until filter paper detached from grid, followed by immediate plunging. | ||||||||||||
| Details | B41 SOSIP.664 was purified by size exclusion chromatography, and concentrated prior to grid application |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated magnification: 38168 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 4.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 22500 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 4.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 22500 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Digitization - Sampling interval: 0.000131 µm / Digitization - Frames/image: 1-35 / Number real images: 2042 / Average exposure time: 7.0 sec. / Average electron dose: 41.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)