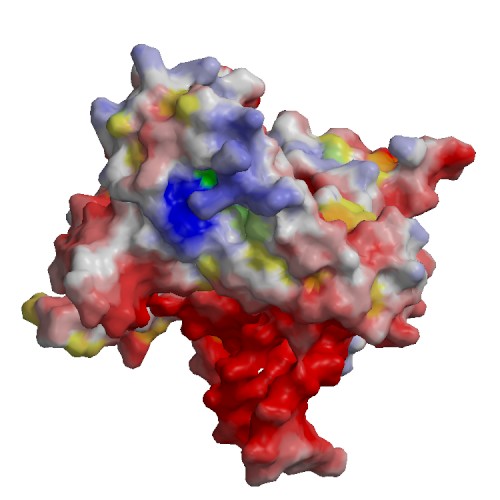
Functional site
| 1) | chain | A |
| residue | 179 | |
| type | ||
| sequence | G | |
| description | binding site for residue DZ4 A 401 | |
| source | : AC1 | |
| 2) | chain | A |
| residue | 180 | |
| type | ||
| sequence | S | |
| description | binding site for residue DZ4 A 401 | |
| source | : AC1 | |
| 3) | chain | A |
| residue | 183 | |
| type | ||
| sequence | R | |
| description | binding site for residue DZ4 A 401 | |
| source | : AC1 | |
| 4) | chain | A |
| residue | 188 | |
| type | ||
| sequence | S | |
| description | binding site for residue DZ4 A 401 | |
| source | : AC1 | |
| 5) | chain | A |
| residue | 189 | |
| type | ||
| sequence | G | |
| description | binding site for residue DZ4 A 401 | |
| source | : AC1 | |
| 6) | chain | A |
| residue | 190 | |
| type | ||
| sequence | D | |
| description | binding site for residue DZ4 A 401 | |
| source | : AC1 | |
| 7) | chain | A |
| residue | 192 | |
| type | ||
| sequence | D | |
| description | binding site for residue DZ4 A 401 | |
| source | : AC1 | |
| 8) | chain | A |
| residue | 271 | |
| type | ||
| sequence | Y | |
| description | binding site for residue DZ4 A 401 | |
| source | : AC1 | |
| 9) | chain | A |
| residue | 272 | |
| type | ||
| sequence | F | |
| description | binding site for residue DZ4 A 401 | |
| source | : AC1 | |
| 10) | chain | A |
| residue | 274 | |
| type | ||
| sequence | G | |
| description | binding site for residue DZ4 A 401 | |
| source | : AC1 | |
| 11) | chain | A |
| residue | 276 | |
| type | ||
| sequence | D | |
| description | binding site for residue DZ4 A 401 | |
| source | : AC1 | |
| 12) | chain | A |
| residue | 279 | |
| type | ||
| sequence | N | |
| description | binding site for residue DZ4 A 401 | |
| source | : AC1 | |
| 13) | chain | A |
| residue | 280 | |
| type | ||
| sequence | K | |
| description | binding site for residue DZ4 A 401 | |
| source | : AC1 | |
| 14) | chain | P |
| residue | 10 | |
| type | ||
| sequence | A | |
| description | binding site for residue DZ4 A 401 | |
| source | : AC1 | |
| 15) | chain | A |
| residue | 190 | |
| type | ||
| sequence | D | |
| description | binding site for residue MN A 402 | |
| source | : AC2 | |
| 16) | chain | A |
| residue | 192 | |
| type | ||
| sequence | D | |
| description | binding site for residue MN A 402 | |
| source | : AC2 | |
| 17) | chain | A |
| residue | 190 | |
| type | ||
| sequence | D | |
| description | binding site for residue MN A 403 | |
| source | : AC3 | |
| 18) | chain | A |
| residue | 192 | |
| type | ||
| sequence | D | |
| description | binding site for residue MN A 403 | |
| source | : AC3 | |
| 19) | chain | P |
| residue | 10 | |
| type | ||
| sequence | A | |
| description | binding site for residue MN A 403 | |
| source | : AC3 | |
| 20) | chain | A |
| residue | 101 | |
| type | ||
| sequence | T | |
| description | binding site for residue NA A 404 | |
| source | : AC4 | |
| 21) | chain | A |
| residue | 103 | |
| type | ||
| sequence | V | |
| description | binding site for residue NA A 404 | |
| source | : AC4 | |
| 22) | chain | A |
| residue | 106 | |
| type | ||
| sequence | I | |
| description | binding site for residue NA A 404 | |
| source | : AC4 | |
| 23) | chain | P |
| residue | 9 | |
| type | ||
| sequence | G | |
| description | binding site for residue NA A 404 | |
| source | : AC4 | |
| 24) | chain | A |
| residue | 60 | |
| type | ||
| sequence | K | |
| description | binding site for residue NA A 405 | |
| source | : AC5 | |
| 25) | chain | A |
| residue | 62 | |
| type | ||
| sequence | L | |
| description | binding site for residue NA A 405 | |
| source | : AC5 | |
| 26) | chain | A |
| residue | 65 | |
| type | ||
| sequence | V | |
| description | binding site for residue NA A 405 | |
| source | : AC5 | |
| 27) | chain | D |
| residue | 3 | |
| type | ||
| sequence | C | |
| description | binding site for residue NA A 405 | |
| source | : AC5 | |
| 28) | chain | A |
| residue | 72 | |
| type | MOD_RES | |
| sequence | K | |
| description | N6-acetyllysine => ECO:0000250|UniProtKB:Q8K409 | |
| source | Swiss-Prot : SWS_FT_FI6 | |
| 29) | chain | A |
| residue | 83 | |
| type | MOD_RES | |
| sequence | R | |
| description | Omega-N-methylarginine; by PRMT6 => ECO:0000269|PubMed:16600869 | |
| source | Swiss-Prot : SWS_FT_FI7 | |
| 30) | chain | A |
| residue | 152 | |
| type | MOD_RES | |
| sequence | R | |
| description | Omega-N-methylarginine; by PRMT6 => ECO:0000269|PubMed:16600869 | |
| source | Swiss-Prot : SWS_FT_FI7 | |
| 31) | chain | A |
| residue | 41 | |
| type | CROSSLNK | |
| sequence | K | |
| description | Glycyl lysine isopeptide (Lys-Gly) (interchain with G-Cter in ubiquitin) => ECO:0000269|PubMed:19713937, ECO:0000269|PubMed:21362556 | |
| source | Swiss-Prot : SWS_FT_FI8 | |
| 32) | chain | A |
| residue | 61 | |
| type | CROSSLNK | |
| sequence | K | |
| description | Glycyl lysine isopeptide (Lys-Gly) (interchain with G-Cter in ubiquitin) => ECO:0000269|PubMed:19713937, ECO:0000269|PubMed:21362556 | |
| source | Swiss-Prot : SWS_FT_FI8 | |
| 33) | chain | A |
| residue | 81 | |
| type | CROSSLNK | |
| sequence | K | |
| description | Glycyl lysine isopeptide (Lys-Gly) (interchain with G-Cter in ubiquitin) => ECO:0000269|PubMed:19713937, ECO:0000269|PubMed:21362556 | |
| source | Swiss-Prot : SWS_FT_FI8 | |
| 34) | chain | A |
| residue | 179-198 | |
| type | prosite | |
| sequence | GSFRRGAESSGDMDVLLTHP | |
| description | DNA_POLYMERASE_X DNA polymerase family X signature. GSFrRGaesSgDMDVLLthP | |
| source | prosite : PS00522 | |
| 35) | chain | A |
| residue | 72 | |
| type | ACT_SITE | |
| sequence | K | |
| description | Nucleophile; Schiff-base intermediate with DNA; for 5'-dRP lyase activity => ECO:0000269|PubMed:9572863 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 36) | chain | A |
| residue | 101 | |
| type | BINDING | |
| sequence | T | |
| description | BINDING => ECO:0000269|PubMed:12517346, ECO:0000269|PubMed:8841120, ECO:0000269|PubMed:9287163, ECO:0007744|PDB:1BPX, ECO:0007744|PDB:1BPZ, ECO:0007744|PDB:1MQ3, ECO:0007744|PDB:1ZQO, ECO:0007744|PDB:1ZQQ | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 37) | chain | A |
| residue | 103 | |
| type | BINDING | |
| sequence | V | |
| description | BINDING => ECO:0000269|PubMed:12517346, ECO:0000269|PubMed:8841120, ECO:0000269|PubMed:9287163, ECO:0007744|PDB:1BPX, ECO:0007744|PDB:1BPZ, ECO:0007744|PDB:1MQ3, ECO:0007744|PDB:1ZQO, ECO:0007744|PDB:1ZQQ | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 38) | chain | A |
| residue | 106 | |
| type | BINDING | |
| sequence | I | |
| description | BINDING => ECO:0000269|PubMed:12517346, ECO:0000269|PubMed:8841120, ECO:0000269|PubMed:9287163, ECO:0007744|PDB:1BPX, ECO:0007744|PDB:1BPZ, ECO:0007744|PDB:1MQ3, ECO:0007744|PDB:1ZQO, ECO:0007744|PDB:1ZQQ | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 39) | chain | A |
| residue | 60 | |
| type | BINDING | |
| sequence | K | |
| description | BINDING => ECO:0000269|PubMed:12517346, ECO:0000269|PubMed:8841120, ECO:0000269|PubMed:9287163, ECO:0007744|PDB:1BPX, ECO:0007744|PDB:1BPZ, ECO:0007744|PDB:1MQ3, ECO:0007744|PDB:1ZQO, ECO:0007744|PDB:1ZQQ | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 40) | chain | A |
| residue | 62 | |
| type | BINDING | |
| sequence | L | |
| description | BINDING => ECO:0000269|PubMed:12517346, ECO:0000269|PubMed:8841120, ECO:0000269|PubMed:9287163, ECO:0007744|PDB:1BPX, ECO:0007744|PDB:1BPZ, ECO:0007744|PDB:1MQ3, ECO:0007744|PDB:1ZQO, ECO:0007744|PDB:1ZQQ | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 41) | chain | A |
| residue | 65 | |
| type | BINDING | |
| sequence | V | |
| description | BINDING => ECO:0000269|PubMed:12517346, ECO:0000269|PubMed:8841120, ECO:0000269|PubMed:9287163, ECO:0007744|PDB:1BPX, ECO:0007744|PDB:1BPZ, ECO:0007744|PDB:1MQ3, ECO:0007744|PDB:1ZQO, ECO:0007744|PDB:1ZQQ | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 42) | chain | A |
| residue | 180 | |
| type | BINDING | |
| sequence | S | |
| description | BINDING => ECO:0000269|PubMed:8841119, ECO:0007744|PDB:8ICW, ECO:0007744|PDB:8ICX, ECO:0007744|PDB:8ICY | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 43) | chain | A |
| residue | 189 | |
| type | BINDING | |
| sequence | G | |
| description | BINDING => ECO:0000269|PubMed:8841119, ECO:0007744|PDB:8ICW, ECO:0007744|PDB:8ICX, ECO:0007744|PDB:8ICY | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 44) | chain | A |
| residue | 149 | |
| type | BINDING | |
| sequence | R | |
| description | BINDING => ECO:0000269|PubMed:8841119, ECO:0007744|PDB:8ICW, ECO:0007744|PDB:8ICX, ECO:0007744|PDB:8ICY | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 45) | chain | A |
| residue | 183 | |
| type | BINDING | |
| sequence | R | |
| description | BINDING => ECO:0000269|PubMed:8841119, ECO:0007744|PDB:8ICX | |
| source | Swiss-Prot : SWS_FT_FI4 | |
| 46) | chain | A |
| residue | 256 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000269|PubMed:9287163, ECO:0007744|PDB:1BPY | |
| source | Swiss-Prot : SWS_FT_FI5 | |
| 47) | chain | A |
| residue | 192 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000269|PubMed:9287163, ECO:0007744|PDB:1BPY | |
| source | Swiss-Prot : SWS_FT_FI5 | |
| 48) | chain | A |
| residue | 190 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000269|PubMed:9287163, ECO:0007744|PDB:1BPY | |
| source | Swiss-Prot : SWS_FT_FI5 | |