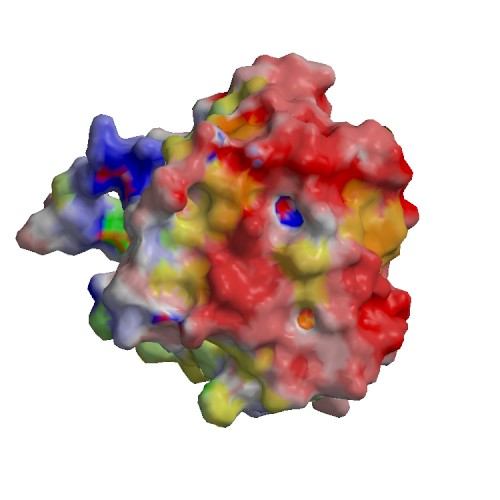
Functional site
| 1) | chain | C |
| residue | 124 | |
| type | ||
| sequence | L | |
| description | binding site for residue HD6 C 401 | |
| source | : AC3 | |
| 2) | chain | C |
| residue | 130 | |
| type | ||
| sequence | W | |
| description | binding site for residue HD6 C 401 | |
| source | : AC3 | |
| 3) | chain | C |
| residue | 133 | |
| type | ||
| sequence | A | |
| description | binding site for residue HD6 C 401 | |
| source | : AC3 | |
| 4) | chain | C |
| residue | 134 | |
| type | ||
| sequence | G | |
| description | binding site for residue HD6 C 401 | |
| source | : AC3 | |
| 5) | chain | C |
| residue | 196 | |
| type | ||
| sequence | C | |
| description | binding site for residue HD6 C 401 | |
| source | : AC3 | |
| 6) | chain | C |
| residue | 198 | |
| type | ||
| sequence | M | |
| description | binding site for residue HD6 C 401 | |
| source | : AC3 | |
| 7) | chain | C |
| residue | 202 | |
| type | ||
| sequence | D | |
| description | binding site for residue HD6 C 401 | |
| source | : AC3 | |
| 8) | chain | C |
| residue | 220 | |
| type | ||
| sequence | P | |
| description | binding site for residue HD6 C 401 | |
| source | : AC3 | |
| 9) | chain | C |
| residue | 222 | |
| type | ||
| sequence | G | |
| description | binding site for residue HD6 C 401 | |
| source | : AC3 | |
| 10) | chain | C |
| residue | 126 | |
| type | BINDING | |
| sequence | H | |
| description | BINDING => ECO:0000305|PubMed:26965057 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 11) | chain | C |
| residue | 164 | |
| type | BINDING | |
| sequence | R | |
| description | BINDING => ECO:0000305|PubMed:26965057 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 12) | chain | C |
| residue | 206 | |
| type | BINDING | |
| sequence | S | |
| description | BINDING => ECO:0000305|PubMed:26965057 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 13) | chain | C |
| residue | 229 | |
| type | BINDING | |
| sequence | E | |
| description | BINDING => ECO:0000305|PubMed:26965057 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 14) | chain | C |
| residue | 126 | |
| type | ACT_SITE | |
| sequence | H | |
| description | Proton acceptor => ECO:0000305|PubMed:26965057, ECO:0000305|PubMed:29185694 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 15) | chain | C |
| residue | 200 | |
| type | ACT_SITE | |
| sequence | E | |
| description | ACT_SITE => ECO:0000305|PubMed:26965057, ECO:0000305|PubMed:29185694 | |
| source | Swiss-Prot : SWS_FT_FI2 | |