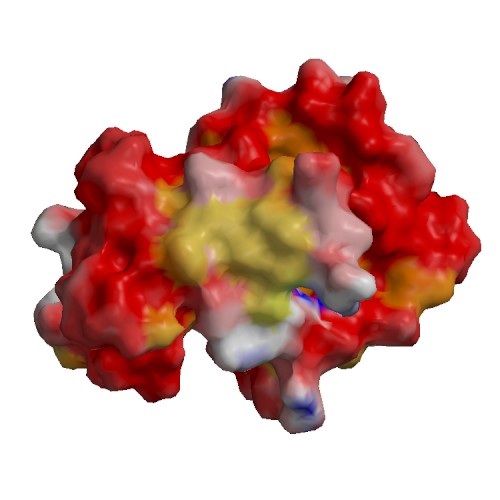
Functional site
| 1) | chain | A |
| residue | 20 | |
| type | ||
| sequence | D | |
| description | BINDING SITE FOR RESIDUE CA A 149 | |
| source | : AC1 | |
| 2) | chain | A |
| residue | 22 | |
| type | ||
| sequence | D | |
| description | BINDING SITE FOR RESIDUE CA A 149 | |
| source | : AC1 | |
| 3) | chain | A |
| residue | 24 | |
| type | ||
| sequence | D | |
| description | BINDING SITE FOR RESIDUE CA A 149 | |
| source | : AC1 | |
| 4) | chain | A |
| residue | 26 | |
| type | ||
| sequence | T | |
| description | BINDING SITE FOR RESIDUE CA A 149 | |
| source | : AC1 | |
| 5) | chain | A |
| residue | 31 | |
| type | ||
| sequence | E | |
| description | BINDING SITE FOR RESIDUE CA A 149 | |
| source | : AC1 | |
| 6) | chain | A |
| residue | 56 | |
| type | ||
| sequence | D | |
| description | BINDING SITE FOR RESIDUE CA A 150 | |
| source | : AC2 | |
| 7) | chain | A |
| residue | 58 | |
| type | ||
| sequence | D | |
| description | BINDING SITE FOR RESIDUE CA A 150 | |
| source | : AC2 | |
| 8) | chain | A |
| residue | 60 | |
| type | ||
| sequence | N | |
| description | BINDING SITE FOR RESIDUE CA A 150 | |
| source | : AC2 | |
| 9) | chain | A |
| residue | 62 | |
| type | ||
| sequence | T | |
| description | BINDING SITE FOR RESIDUE CA A 150 | |
| source | : AC2 | |
| 10) | chain | A |
| residue | 67 | |
| type | ||
| sequence | E | |
| description | BINDING SITE FOR RESIDUE CA A 150 | |
| source | : AC2 | |
| 11) | chain | A |
| residue | 93 | |
| type | ||
| sequence | D | |
| description | BINDING SITE FOR RESIDUE CA A 151 | |
| source | : AC3 | |
| 12) | chain | A |
| residue | 95 | |
| type | ||
| sequence | D | |
| description | BINDING SITE FOR RESIDUE CA A 151 | |
| source | : AC3 | |
| 13) | chain | A |
| residue | 97 | |
| type | ||
| sequence | N | |
| description | BINDING SITE FOR RESIDUE CA A 151 | |
| source | : AC3 | |
| 14) | chain | A |
| residue | 99 | |
| type | ||
| sequence | Y | |
| description | BINDING SITE FOR RESIDUE CA A 151 | |
| source | : AC3 | |
| 15) | chain | A |
| residue | 104 | |
| type | ||
| sequence | E | |
| description | BINDING SITE FOR RESIDUE CA A 151 | |
| source | : AC3 | |
| 16) | chain | A |
| residue | 129 | |
| type | ||
| sequence | D | |
| description | BINDING SITE FOR RESIDUE CA A 152 | |
| source | : AC4 | |
| 17) | chain | A |
| residue | 131 | |
| type | ||
| sequence | D | |
| description | BINDING SITE FOR RESIDUE CA A 152 | |
| source | : AC4 | |
| 18) | chain | A |
| residue | 133 | |
| type | ||
| sequence | D | |
| description | BINDING SITE FOR RESIDUE CA A 152 | |
| source | : AC4 | |
| 19) | chain | A |
| residue | 135 | |
| type | ||
| sequence | Q | |
| description | BINDING SITE FOR RESIDUE CA A 152 | |
| source | : AC4 | |
| 20) | chain | A |
| residue | 140 | |
| type | ||
| sequence | E | |
| description | BINDING SITE FOR RESIDUE CA A 152 | |
| source | : AC4 | |
| 21) | chain | A |
| residue | 11 | |
| type | ||
| sequence | E | |
| description | BINDING SITE FOR RESIDUE SPU A 153 | |
| source | : AC5 | |
| 22) | chain | A |
| residue | 76 | |
| type | ||
| sequence | M | |
| description | BINDING SITE FOR RESIDUE SPU A 153 | |
| source | : AC5 | |
| 23) | chain | A |
| residue | 88 | |
| type | ||
| sequence | A | |
| description | BINDING SITE FOR RESIDUE SPU A 153 | |
| source | : AC5 | |
| 24) | chain | A |
| residue | 92 | |
| type | ||
| sequence | F | |
| description | BINDING SITE FOR RESIDUE SPU A 153 | |
| source | : AC5 | |
| 25) | chain | A |
| residue | 123 | |
| type | ||
| sequence | E | |
| description | BINDING SITE FOR RESIDUE SPU A 153 | |
| source | : AC5 | |
| 26) | chain | A |
| residue | 124 | |
| type | ||
| sequence | M | |
| description | BINDING SITE FOR RESIDUE SPU A 153 | |
| source | : AC5 | |
| 27) | chain | A |
| residue | 127 | |
| type | ||
| sequence | E | |
| description | BINDING SITE FOR RESIDUE SPU A 153 | |
| source | : AC5 | |
| 28) | chain | A |
| residue | 141 | |
| type | ||
| sequence | F | |
| description | BINDING SITE FOR RESIDUE SPU A 153 | |
| source | : AC5 | |
| 29) | chain | A |
| residue | 144 | |
| type | ||
| sequence | M | |
| description | BINDING SITE FOR RESIDUE SPU A 153 | |
| source | : AC5 | |
| 30) | chain | A |
| residue | 145 | |
| type | ||
| sequence | M | |
| description | BINDING SITE FOR RESIDUE SPU A 153 | |
| source | : AC5 | |
| 31) | chain | A |
| residue | 11 | |
| type | ||
| sequence | E | |
| description | BINDING SITE FOR RESIDUE SPU A 154 | |
| source | : AC6 | |
| 32) | chain | A |
| residue | 39 | |
| type | ||
| sequence | L | |
| description | BINDING SITE FOR RESIDUE SPU A 154 | |
| source | : AC6 | |
| 33) | chain | A |
| residue | 87 | |
| type | ||
| sequence | E | |
| description | BINDING SITE FOR RESIDUE SPU A 154 | |
| source | : AC6 | |
| 34) | chain | A |
| residue | 124 | |
| type | ||
| sequence | M | |
| description | BINDING SITE FOR RESIDUE SPU A 154 | |
| source | : AC6 | |
| 35) | chain | A |
| residue | 12 | |
| type | ||
| sequence | F | |
| description | BINDING SITE FOR RESIDUE SPU A 155 | |
| source | : AC7 | |
| 36) | chain | A |
| residue | 15 | |
| type | ||
| sequence | A | |
| description | BINDING SITE FOR RESIDUE SPU A 155 | |
| source | : AC7 | |
| 37) | chain | A |
| residue | 32 | |
| type | ||
| sequence | L | |
| description | BINDING SITE FOR RESIDUE SPU A 155 | |
| source | : AC7 | |
| 38) | chain | A |
| residue | 51 | |
| type | ||
| sequence | M | |
| description | BINDING SITE FOR RESIDUE SPU A 155 | |
| source | : AC7 | |
| 39) | chain | A |
| residue | 54 | |
| type | ||
| sequence | E | |
| description | BINDING SITE FOR RESIDUE SPU A 155 | |
| source | : AC7 | |
| 40) | chain | A |
| residue | 55 | |
| type | ||
| sequence | V | |
| description | BINDING SITE FOR RESIDUE SPU A 155 | |
| source | : AC7 | |
| 41) | chain | A |
| residue | 71 | |
| type | ||
| sequence | M | |
| description | BINDING SITE FOR RESIDUE SPU A 155 | |
| source | : AC7 | |
| 42) | chain | A |
| residue | 72 | |
| type | ||
| sequence | M | |
| description | BINDING SITE FOR RESIDUE SPU A 155 | |
| source | : AC7 | |
| 43) | chain | A |
| residue | 145 | |
| type | ||
| sequence | M | |
| description | BINDING SITE FOR RESIDUE SPU A 156 | |
| source | : AC8 | |
| 44) | chain | A |
| residue | 20-32 | |
| type | prosite | |
| sequence | DKDGDGTITTKEL | |
| description | EF_HAND_1 EF-hand calcium-binding domain. DKDGDGTITtkEL | |
| source | prosite : PS00018 | |
| 45) | chain | A |
| residue | 56-68 | |
| type | prosite | |
| sequence | DADGNGTIDFPEF | |
| description | EF_HAND_1 EF-hand calcium-binding domain. DKDGDGTITtkEL | |
| source | prosite : PS00018 | |
| 46) | chain | A |
| residue | 93-105 | |
| type | prosite | |
| sequence | DKDGNGYISAAEL | |
| description | EF_HAND_1 EF-hand calcium-binding domain. DKDGDGTITtkEL | |
| source | prosite : PS00018 | |
| 47) | chain | A |
| residue | 129-141 | |
| type | prosite | |
| sequence | DIDGDGQVNYEEF | |
| description | EF_HAND_1 EF-hand calcium-binding domain. DKDGDGTITtkEL | |
| source | prosite : PS00018 | |
| 48) | chain | A |
| residue | 101 | |
| type | MOD_RES | |
| sequence | S | |
| description | Phosphoserine => ECO:0000250|UniProtKB:P0DP23 | |
| source | Swiss-Prot : SWS_FT_FI5 | |
| 49) | chain | A |
| residue | 81 | |
| type | MOD_RES | |
| sequence | S | |
| description | Phosphoserine => ECO:0000250|UniProtKB:P0DP23 | |
| source | Swiss-Prot : SWS_FT_FI5 | |
| 50) | chain | A |
| residue | 94 | |
| type | MOD_RES | |
| sequence | K | |
| description | N6-acetyllysine => ECO:0000250|UniProtKB:P0DP23 | |
| source | Swiss-Prot : SWS_FT_FI6 | |
| 51) | chain | A |
| residue | 138 | |
| type | MOD_RES | |
| sequence | Y | |
| description | Phosphotyrosine => ECO:0000250|UniProtKB:P0DP23 | |
| source | Swiss-Prot : SWS_FT_FI7 | |
| 52) | chain | A |
| residue | 99 | |
| type | MOD_RES | |
| sequence | Y | |
| description | Phosphotyrosine => ECO:0000250|UniProtKB:P0DP23 | |
| source | Swiss-Prot : SWS_FT_FI7 | |
| 53) | chain | A |
| residue | 110 | |
| type | MOD_RES | |
| sequence | T | |
| description | Phosphothreonine => ECO:0000250|UniProtKB:P0DP23 | |
| source | Swiss-Prot : SWS_FT_FI8 | |
| 54) | chain | A |
| residue | 115 | |
| type | MOD_RES | |
| sequence | K | |
| description | N6-methyllysine; alternate => ECO:0000250|UniProtKB:P0DP23 | |
| source | Swiss-Prot : SWS_FT_FI9 | |
| 55) | chain | A |
| residue | 21 | |
| type | CROSSLNK | |
| sequence | K | |
| description | Glycyl lysine isopeptide (Lys-Gly) (interchain with G-Cter in ubiquitin); alternate => ECO:0000269|PubMed:9716384 | |
| source | Swiss-Prot : SWS_FT_FI10 | |
| 56) | chain | A |
| residue | 67 | |
| type | BINDING | |
| sequence | E | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 57) | chain | A |
| residue | 93 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 58) | chain | A |
| residue | 95 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 59) | chain | A |
| residue | 97 | |
| type | BINDING | |
| sequence | N | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 60) | chain | A |
| residue | 99 | |
| type | BINDING | |
| sequence | Y | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 61) | chain | A |
| residue | 104 | |
| type | BINDING | |
| sequence | E | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 62) | chain | A |
| residue | 129 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 63) | chain | A |
| residue | 131 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 64) | chain | A |
| residue | 133 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 65) | chain | A |
| residue | 135 | |
| type | BINDING | |
| sequence | Q | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 66) | chain | A |
| residue | 140 | |
| type | BINDING | |
| sequence | E | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 67) | chain | A |
| residue | 56 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 68) | chain | A |
| residue | 58 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 69) | chain | A |
| residue | 60 | |
| type | BINDING | |
| sequence | N | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 70) | chain | A |
| residue | 62 | |
| type | BINDING | |
| sequence | T | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 71) | chain | A |
| residue | 20 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 72) | chain | A |
| residue | 22 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 73) | chain | A |
| residue | 24 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 74) | chain | A |
| residue | 26 | |
| type | BINDING | |
| sequence | T | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 75) | chain | A |
| residue | 31 | |
| type | BINDING | |
| sequence | E | |
| description | BINDING => ECO:0000255|PROSITE-ProRule:PRU00448 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 76) | chain | A |
| residue | 21 | |
| type | MOD_RES | |
| sequence | K | |
| description | N6-acetyllysine; alternate => ECO:0000250|UniProtKB:P0DP23 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 77) | chain | A |
| residue | 44 | |
| type | MOD_RES | |
| sequence | T | |
| description | Phosphothreonine; by CaMK4 => ECO:0000250|UniProtKB:P0DP29 | |
| source | Swiss-Prot : SWS_FT_FI4 | |