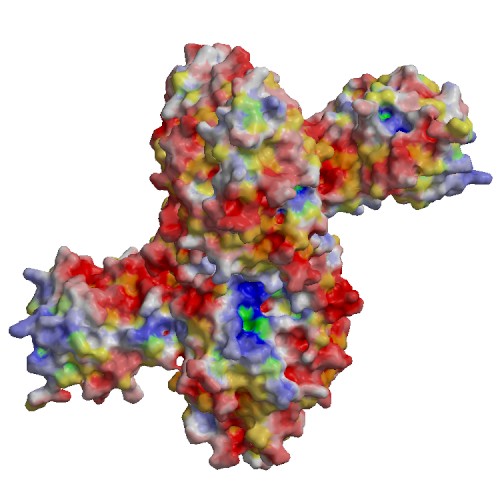
Functional site
| 1) | chain | E |
| residue | 184 | |
| type | CARBOHYD | |
| sequence | N | |
| description | N-linked (GlcNAc...) asparagine => ECO:0000255 | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 2) | chain | F |
| residue | 184 | |
| type | CARBOHYD | |
| sequence | N | |
| description | N-linked (GlcNAc...) asparagine => ECO:0000255 | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 3) | chain | B |
| residue | 332 | |
| type | CARBOHYD | |
| sequence | Y | |
| description | N-linked (GlcNAc...) asparagine => ECO:0000255 | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 4) | chain | B |
| residue | 372 | |
| type | CARBOHYD | |
| sequence | Y | |
| description | N-linked (GlcNAc...) asparagine => ECO:0000255 | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 5) | chain | A |
| residue | 576 | |
| type | CARBOHYD | |
| sequence | V | |
| description | N-linked (GlcNAc...) asparagine => ECO:0000269|PubMed:18084303 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 6) | chain | B |
| residue | 576 | |
| type | CARBOHYD | |
| sequence | V | |
| description | N-linked (GlcNAc...) asparagine => ECO:0000269|PubMed:18084303 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 7) | chain | E |
| residue | 137 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000269|PubMed:18084303 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 8) | chain | E |
| residue | 154 | |
| type | BINDING | |
| sequence | V | |
| description | BINDING => ECO:0000269|PubMed:18084303 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 9) | chain | E |
| residue | 266 | |
| type | BINDING | |
| sequence | L | |
| description | BINDING => ECO:0000269|PubMed:18084303 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 10) | chain | E |
| residue | 268 | |
| type | BINDING | |
| sequence | V | |
| description | BINDING => ECO:0000269|PubMed:18084303 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 11) | chain | F |
| residue | 137 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000269|PubMed:18084303 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 12) | chain | F |
| residue | 154 | |
| type | BINDING | |
| sequence | V | |
| description | BINDING => ECO:0000269|PubMed:18084303 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 13) | chain | F |
| residue | 266 | |
| type | BINDING | |
| sequence | L | |
| description | BINDING => ECO:0000269|PubMed:18084303 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 14) | chain | F |
| residue | 268 | |
| type | BINDING | |
| sequence | V | |
| description | BINDING => ECO:0000269|PubMed:18084303 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 15) | chain | A |
| residue | 151-161 | |
| type | prosite | |
| sequence | EDCLYLNIYVP | |
| description | CARBOXYLESTERASE_B_2 Carboxylesterases type-B signature 2. EDCLYLNIYvP | |
| source | prosite : PS00941 | |