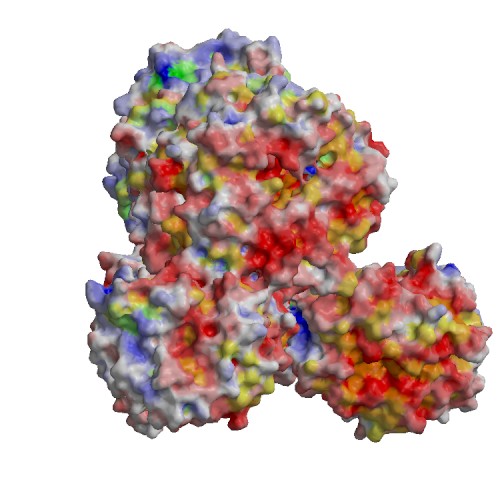
Functional site
| 1) | chain | C |
| residue | 547 | |
| type | catalytic | |
| sequence | Y | |
| description | 169 | |
| source | MCSA : MCSA3 | |
| 2) | chain | C |
| residue | 630 | |
| type | catalytic | |
| sequence | S | |
| description | 169 | |
| source | MCSA : MCSA3 | |
| 3) | chain | C |
| residue | 631 | |
| type | catalytic | |
| sequence | Y | |
| description | 169 | |
| source | MCSA : MCSA3 | |
| 4) | chain | C |
| residue | 708 | |
| type | catalytic | |
| sequence | D | |
| description | 169 | |
| source | MCSA : MCSA3 | |
| 5) | chain | C |
| residue | 740 | |
| type | catalytic | |
| sequence | H | |
| description | 169 | |
| source | MCSA : MCSA3 | |
| 6) | chain | D |
| residue | 547 | |
| type | catalytic | |
| sequence | Y | |
| description | 169 | |
| source | MCSA : MCSA4 | |
| 7) | chain | D |
| residue | 630 | |
| type | catalytic | |
| sequence | S | |
| description | 169 | |
| source | MCSA : MCSA4 | |
| 8) | chain | D |
| residue | 631 | |
| type | catalytic | |
| sequence | Y | |
| description | 169 | |
| source | MCSA : MCSA4 | |
| 9) | chain | D |
| residue | 708 | |
| type | catalytic | |
| sequence | D | |
| description | 169 | |
| source | MCSA : MCSA4 | |
| 10) | chain | D |
| residue | 740 | |
| type | catalytic | |
| sequence | H | |
| description | 169 | |
| source | MCSA : MCSA4 | |
| 11) | chain | D |
| residue | 630 | |
| type | ACT_SITE | |
| sequence | S | |
| description | Proton donor => ECO:0000250|UniProtKB:P03958 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 12) | chain | D |
| residue | 708 | |
| type | ACT_SITE | |
| sequence | D | |
| description | Proton donor => ECO:0000250|UniProtKB:P03958 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 13) | chain | D |
| residue | 740 | |
| type | ACT_SITE | |
| sequence | H | |
| description | Proton donor => ECO:0000250|UniProtKB:P03958 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 14) | chain | G |
| residue | 218 | |
| type | ACT_SITE | |
| sequence | V | |
| description | Proton donor => ECO:0000250|UniProtKB:P03958 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 15) | chain | H |
| residue | 218 | |
| type | ACT_SITE | |
| sequence | V | |
| description | Proton donor => ECO:0000250|UniProtKB:P03958 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 16) | chain | C |
| residue | 630 | |
| type | ACT_SITE | |
| sequence | S | |
| description | Proton donor => ECO:0000250|UniProtKB:P03958 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 17) | chain | C |
| residue | 708 | |
| type | ACT_SITE | |
| sequence | D | |
| description | Proton donor => ECO:0000250|UniProtKB:P03958 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 18) | chain | C |
| residue | 740 | |
| type | ACT_SITE | |
| sequence | H | |
| description | Proton donor => ECO:0000250|UniProtKB:P03958 | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 19) | chain | G |
| residue | 18 | |
| type | BINDING | |
| sequence | L | |
| description | BINDING => ECO:0000269|PubMed:12554940, ECO:0000269|PubMed:14709046, ECO:0000269|PubMed:15139750, ECO:0000269|PubMed:15213224, ECO:0000269|PubMed:15239652, ECO:0000269|PubMed:15695814, ECO:0000269|PubMed:16033254, ECO:0000269|PubMed:16060665, ECO:0000269|PubMed:18549808, ECO:0007744|PDB:1KRM, ECO:0007744|PDB:1NDV, ECO:0007744|PDB:1NDW, ECO:0007744|PDB:1NDY, ECO:0007744|PDB:1NDZ, ECO:0007744|PDB:1O5R, ECO:0007744|PDB:1QXL, ECO:0007744|PDB:1VFL | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 20) | chain | G |
| residue | 215 | |
| type | BINDING | |
| sequence | A | |
| description | BINDING => ECO:0000269|PubMed:12554940, ECO:0000269|PubMed:14709046, ECO:0000269|PubMed:15139750, ECO:0000269|PubMed:15213224, ECO:0000269|PubMed:15239652, ECO:0000269|PubMed:15695814, ECO:0000269|PubMed:16033254, ECO:0000269|PubMed:16060665, ECO:0000269|PubMed:18549808, ECO:0007744|PDB:1KRM, ECO:0007744|PDB:1NDV, ECO:0007744|PDB:1NDW, ECO:0007744|PDB:1NDY, ECO:0007744|PDB:1NDZ, ECO:0007744|PDB:1O5R, ECO:0007744|PDB:1QXL, ECO:0007744|PDB:1VFL | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 21) | chain | G |
| residue | 296 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000269|PubMed:12554940, ECO:0000269|PubMed:14709046, ECO:0000269|PubMed:15139750, ECO:0000269|PubMed:15213224, ECO:0000269|PubMed:15239652, ECO:0000269|PubMed:15695814, ECO:0000269|PubMed:16033254, ECO:0000269|PubMed:16060665, ECO:0000269|PubMed:18549808, ECO:0007744|PDB:1KRM, ECO:0007744|PDB:1NDV, ECO:0007744|PDB:1NDW, ECO:0007744|PDB:1NDY, ECO:0007744|PDB:1NDZ, ECO:0007744|PDB:1O5R, ECO:0007744|PDB:1QXL, ECO:0007744|PDB:1VFL | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 22) | chain | H |
| residue | 16 | |
| type | BINDING | |
| sequence | V | |
| description | BINDING => ECO:0000269|PubMed:12554940, ECO:0000269|PubMed:14709046, ECO:0000269|PubMed:15139750, ECO:0000269|PubMed:15213224, ECO:0000269|PubMed:15239652, ECO:0000269|PubMed:15695814, ECO:0000269|PubMed:16033254, ECO:0000269|PubMed:16060665, ECO:0000269|PubMed:18549808, ECO:0007744|PDB:1KRM, ECO:0007744|PDB:1NDV, ECO:0007744|PDB:1NDW, ECO:0007744|PDB:1NDY, ECO:0007744|PDB:1NDZ, ECO:0007744|PDB:1O5R, ECO:0007744|PDB:1QXL, ECO:0007744|PDB:1VFL | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 23) | chain | H |
| residue | 18 | |
| type | BINDING | |
| sequence | L | |
| description | BINDING => ECO:0000269|PubMed:12554940, ECO:0000269|PubMed:14709046, ECO:0000269|PubMed:15139750, ECO:0000269|PubMed:15213224, ECO:0000269|PubMed:15239652, ECO:0000269|PubMed:15695814, ECO:0000269|PubMed:16033254, ECO:0000269|PubMed:16060665, ECO:0000269|PubMed:18549808, ECO:0007744|PDB:1KRM, ECO:0007744|PDB:1NDV, ECO:0007744|PDB:1NDW, ECO:0007744|PDB:1NDY, ECO:0007744|PDB:1NDZ, ECO:0007744|PDB:1O5R, ECO:0007744|PDB:1QXL, ECO:0007744|PDB:1VFL | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 24) | chain | H |
| residue | 215 | |
| type | BINDING | |
| sequence | A | |
| description | BINDING => ECO:0000269|PubMed:12554940, ECO:0000269|PubMed:14709046, ECO:0000269|PubMed:15139750, ECO:0000269|PubMed:15213224, ECO:0000269|PubMed:15239652, ECO:0000269|PubMed:15695814, ECO:0000269|PubMed:16033254, ECO:0000269|PubMed:16060665, ECO:0000269|PubMed:18549808, ECO:0007744|PDB:1KRM, ECO:0007744|PDB:1NDV, ECO:0007744|PDB:1NDW, ECO:0007744|PDB:1NDY, ECO:0007744|PDB:1NDZ, ECO:0007744|PDB:1O5R, ECO:0007744|PDB:1QXL, ECO:0007744|PDB:1VFL | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 25) | chain | H |
| residue | 296 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000269|PubMed:12554940, ECO:0000269|PubMed:14709046, ECO:0000269|PubMed:15139750, ECO:0000269|PubMed:15213224, ECO:0000269|PubMed:15239652, ECO:0000269|PubMed:15695814, ECO:0000269|PubMed:16033254, ECO:0000269|PubMed:16060665, ECO:0000269|PubMed:18549808, ECO:0007744|PDB:1KRM, ECO:0007744|PDB:1NDV, ECO:0007744|PDB:1NDW, ECO:0007744|PDB:1NDY, ECO:0007744|PDB:1NDZ, ECO:0007744|PDB:1O5R, ECO:0007744|PDB:1QXL, ECO:0007744|PDB:1VFL | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 26) | chain | G |
| residue | 16 | |
| type | BINDING | |
| sequence | V | |
| description | BINDING => ECO:0000269|PubMed:12554940, ECO:0000269|PubMed:14709046, ECO:0000269|PubMed:15139750, ECO:0000269|PubMed:15213224, ECO:0000269|PubMed:15239652, ECO:0000269|PubMed:15695814, ECO:0000269|PubMed:16033254, ECO:0000269|PubMed:16060665, ECO:0000269|PubMed:18549808, ECO:0007744|PDB:1KRM, ECO:0007744|PDB:1NDV, ECO:0007744|PDB:1NDW, ECO:0007744|PDB:1NDY, ECO:0007744|PDB:1NDZ, ECO:0007744|PDB:1O5R, ECO:0007744|PDB:1QXL, ECO:0007744|PDB:1VFL | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 27) | chain | G |
| residue | 20 | |
| type | BINDING | |
| sequence | G | |
| description | BINDING => ECO:0000269|PubMed:12554940, ECO:0000269|PubMed:14709046, ECO:0000269|PubMed:15139750, ECO:0000269|PubMed:15239652, ECO:0000269|PubMed:16033254, ECO:0000269|PubMed:16060665, ECO:0000269|PubMed:18549808, ECO:0007744|PDB:1NDW, ECO:0007744|PDB:1NDY, ECO:0007744|PDB:1NDZ, ECO:0007744|PDB:1O5R, ECO:0007744|PDB:1QXL | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 28) | chain | H |
| residue | 20 | |
| type | BINDING | |
| sequence | G | |
| description | BINDING => ECO:0000269|PubMed:12554940, ECO:0000269|PubMed:14709046, ECO:0000269|PubMed:15139750, ECO:0000269|PubMed:15239652, ECO:0000269|PubMed:16033254, ECO:0000269|PubMed:16060665, ECO:0000269|PubMed:18549808, ECO:0007744|PDB:1NDW, ECO:0007744|PDB:1NDY, ECO:0007744|PDB:1NDZ, ECO:0007744|PDB:1O5R, ECO:0007744|PDB:1QXL | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 29) | chain | G |
| residue | 185 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000269|PubMed:14709046, ECO:0000269|PubMed:16060665, ECO:0007744|PDB:1NDY, ECO:0007744|PDB:1WXY | |
| source | Swiss-Prot : SWS_FT_FI4 | |
| 30) | chain | H |
| residue | 185 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000269|PubMed:14709046, ECO:0000269|PubMed:16060665, ECO:0007744|PDB:1NDY, ECO:0007744|PDB:1WXY | |
| source | Swiss-Prot : SWS_FT_FI4 | |
| 31) | chain | G |
| residue | 297 | |
| type | BINDING | |
| sequence | P | |
| description | BINDING => ECO:0000269|PubMed:12554940, ECO:0000269|PubMed:14709046, ECO:0000269|PubMed:15139750, ECO:0000269|PubMed:15239652, ECO:0000269|PubMed:16033254, ECO:0000269|PubMed:18549808, ECO:0007744|PDB:1NDW, ECO:0007744|PDB:1NDY, ECO:0007744|PDB:1NDZ, ECO:0007744|PDB:1O5R, ECO:0007744|PDB:1QXL | |
| source | Swiss-Prot : SWS_FT_FI5 | |
| 32) | chain | H |
| residue | 297 | |
| type | BINDING | |
| sequence | P | |
| description | BINDING => ECO:0000269|PubMed:12554940, ECO:0000269|PubMed:14709046, ECO:0000269|PubMed:15139750, ECO:0000269|PubMed:15239652, ECO:0000269|PubMed:16033254, ECO:0000269|PubMed:18549808, ECO:0007744|PDB:1NDW, ECO:0007744|PDB:1NDY, ECO:0007744|PDB:1NDZ, ECO:0007744|PDB:1O5R, ECO:0007744|PDB:1QXL | |
| source | Swiss-Prot : SWS_FT_FI5 | |
| 33) | chain | G |
| residue | 59 | |
| type | SITE | |
| sequence | P | |
| description | Important for interaction with adenosine receptors and increasing their affinity for agonists => ECO:0000250|UniProtKB:P00813 | |
| source | Swiss-Prot : SWS_FT_FI6 | |
| 34) | chain | G |
| residue | 63 | |
| type | SITE | |
| sequence | A | |
| description | Important for interaction with adenosine receptors and increasing their affinity for agonists => ECO:0000250|UniProtKB:P00813 | |
| source | Swiss-Prot : SWS_FT_FI6 | |
| 35) | chain | H |
| residue | 59 | |
| type | SITE | |
| sequence | P | |
| description | Important for interaction with adenosine receptors and increasing their affinity for agonists => ECO:0000250|UniProtKB:P00813 | |
| source | Swiss-Prot : SWS_FT_FI6 | |
| 36) | chain | H |
| residue | 63 | |
| type | SITE | |
| sequence | A | |
| description | Important for interaction with adenosine receptors and increasing their affinity for agonists => ECO:0000250|UniProtKB:P00813 | |
| source | Swiss-Prot : SWS_FT_FI6 | |
| 37) | chain | G |
| residue | 239 | |
| type | SITE | |
| sequence | G | |
| description | Important for catalytic activity => ECO:0000250|UniProtKB:P03958 | |
| source | Swiss-Prot : SWS_FT_FI7 | |
| 38) | chain | H |
| residue | 239 | |
| type | SITE | |
| sequence | G | |
| description | Important for catalytic activity => ECO:0000250|UniProtKB:P03958 | |
| source | Swiss-Prot : SWS_FT_FI7 | |
| 39) | chain | G |
| residue | 55 | |
| type | MOD_RES | |
| sequence | P | |
| description | N6-acetyllysine => ECO:0000250|UniProtKB:P00813 | |
| source | Swiss-Prot : SWS_FT_FI9 | |
| 40) | chain | G |
| residue | 233 | |
| type | MOD_RES | |
| sequence | T | |
| description | N6-acetyllysine => ECO:0000250|UniProtKB:P00813 | |
| source | Swiss-Prot : SWS_FT_FI9 | |
| 41) | chain | H |
| residue | 55 | |
| type | MOD_RES | |
| sequence | P | |
| description | N6-acetyllysine => ECO:0000250|UniProtKB:P00813 | |
| source | Swiss-Prot : SWS_FT_FI9 | |
| 42) | chain | H |
| residue | 233 | |
| type | MOD_RES | |
| sequence | T | |
| description | N6-acetyllysine => ECO:0000250|UniProtKB:P00813 | |
| source | Swiss-Prot : SWS_FT_FI9 | |