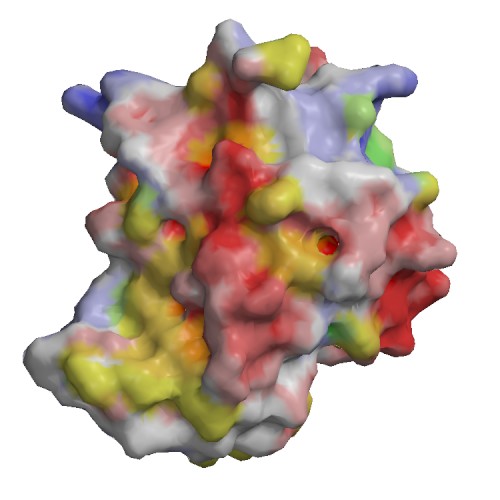
Functional site
| 1) | chain | A |
| residue | 25 | |
| type | ||
| sequence | H | |
| description | BINDING SITE FOR RESIDUE MN A 1001 | |
| source | : AC1 | |
| 2) | chain | A |
| residue | 32 | |
| type | ||
| sequence | H | |
| description | BINDING SITE FOR RESIDUE MN A 1001 | |
| source | : AC1 | |
| 3) | chain | A |
| residue | 69 | |
| type | ||
| sequence | H | |
| description | BINDING SITE FOR RESIDUE MN A 1001 | |
| source | : AC1 | |
| 4) | chain | A |
| residue | 114 | |
| type | ||
| sequence | E | |
| description | BINDING SITE FOR RESIDUE MN A 1001 | |
| source | : AC1 | |
| 5) | chain | A |
| residue | 116 | |
| type | ||
| sequence | E | |
| description | BINDING SITE FOR RESIDUE MN A 1001 | |
| source | : AC1 | |
| 6) | chain | A |
| residue | 25 | |
| type | catalytic | |
| sequence | H | |
| description | 190 | |
| source | MCSA : MCSA1 | |
| 7) | chain | A |
| residue | 32 | |
| type | catalytic | |
| sequence | H | |
| description | 190 | |
| source | MCSA : MCSA1 | |
| 8) | chain | A |
| residue | 67 | |
| type | catalytic | |
| sequence | C | |
| description | 190 | |
| source | MCSA : MCSA1 | |
| 9) | chain | A |
| residue | 69 | |
| type | catalytic | |
| sequence | H | |
| description | 190 | |
| source | MCSA : MCSA1 | |
| 10) | chain | A |
| residue | 87 | |
| type | catalytic | |
| sequence | E | |
| description | 190 | |
| source | MCSA : MCSA1 | |
| 11) | chain | A |
| residue | 104 | |
| type | catalytic | |
| sequence | Y | |
| description | 190 | |
| source | MCSA : MCSA1 | |
| 12) | chain | A |
| residue | 114 | |
| type | catalytic | |
| sequence | E | |
| description | 190 | |
| source | MCSA : MCSA1 | |
| 13) | chain | A |
| residue | 116 | |
| type | catalytic | |
| sequence | E | |
| description | 190 | |
| source | MCSA : MCSA1 | |
| 14) | chain | A |
| residue | 161 | |
| type | catalytic | |
| sequence | W | |
| description | 190 | |
| source | MCSA : MCSA1 | |
| 15) | chain | A |
| residue | 21 | |
| type | BINDING | |
| sequence | K | |
| description | ||
| source | Swiss-Prot : SWS_FT_FI2 | |
| 16) | chain | A |
| residue | 51 | |
| type | BINDING | |
| sequence | R | |
| description | ||
| source | Swiss-Prot : SWS_FT_FI2 | |
| 17) | chain | A |
| residue | 55 | |
| type | BINDING | |
| sequence | K | |
| description | ||
| source | Swiss-Prot : SWS_FT_FI2 | |
| 18) | chain | A |
| residue | 67 | |
| type | BINDING | |
| sequence | C | |
| description | ||
| source | Swiss-Prot : SWS_FT_FI2 | |
| 19) | chain | A |
| residue | 69 | |
| type | BINDING | |
| sequence | H | |
| description | ||
| source | Swiss-Prot : SWS_FT_FI2 | |
| 20) | chain | A |
| residue | 83 | |
| type | BINDING | |
| sequence | R | |
| description | ||
| source | Swiss-Prot : SWS_FT_FI2 | |
| 21) | chain | A |
| residue | 87 | |
| type | BINDING | |
| sequence | E | |
| description | ||
| source | Swiss-Prot : SWS_FT_FI2 | |
| 22) | chain | A |
| residue | 25 | |
| type | BINDING | |
| sequence | H | |
| description | BINDING => ECO:0000269|PubMed:12630859, ECO:0000269|PubMed:15643873 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 23) | chain | A |
| residue | 32 | |
| type | BINDING | |
| sequence | H | |
| description | BINDING => ECO:0000269|PubMed:12630859, ECO:0000269|PubMed:15643873 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 24) | chain | A |
| residue | 114 | |
| type | BINDING | |
| sequence | E | |
| description | BINDING => ECO:0000269|PubMed:12630859, ECO:0000269|PubMed:15643873 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 25) | chain | A |
| residue | 116 | |
| type | BINDING | |
| sequence | E | |
| description | BINDING => ECO:0000269|PubMed:12630859, ECO:0000269|PubMed:15643873 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 26) | chain | A |
| residue | 104 | |
| type | SITE | |
| sequence | Y | |
| description | Essential for catalytic activity | |
| source | Swiss-Prot : SWS_FT_FI4 | |
| 27) | chain | A |
| residue | 67 | |
| type | ACT_SITE | |
| sequence | C | |
| description | ||
| source | Swiss-Prot : SWS_FT_FI1 | |
| 28) | chain | A |
| residue | 116 | |
| type | ACT_SITE | |
| sequence | E | |
| description | ||
| source | Swiss-Prot : SWS_FT_FI1 | |