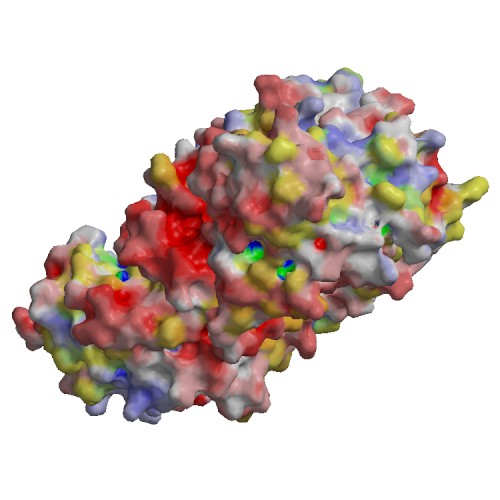
Functional site
| 1) | chain | A |
| residue | 558 | |
| type | MOD_RES | |
| sequence | A | |
| description | N6-acetyllysine => ECO:0000250|UniProtKB:P13020 | |
| source | Swiss-Prot : SWS_FT_FI5 | |
| 2) | chain | A |
| residue | 716 | |
| type | MOD_RES | |
| sequence | P | |
| description | Phosphothreonine => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI6 | |
| 3) | chain | A |
| residue | 66 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000269|PubMed:15215896, ECO:0007744|PDB:1RGI | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 4) | chain | A |
| residue | 328 | |
| type | BINDING | |
| sequence | R | |
| description | BINDING => ECO:0000269|PubMed:15215896, ECO:0007744|PDB:1RGI | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 5) | chain | A |
| residue | 67 | |
| type | BINDING | |
| sequence | A | |
| description | BINDING => ECO:0000269|PubMed:15215896, ECO:0007744|PDB:1RGI | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 6) | chain | A |
| residue | 98 | |
| type | BINDING | |
| sequence | S | |
| description | BINDING => ECO:0000269|PubMed:15215896, ECO:0007744|PDB:1RGI | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 7) | chain | A |
| residue | 110 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000269|PubMed:15215896, ECO:0007744|PDB:1RGI | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 8) | chain | A |
| residue | 115 | |
| type | BINDING | |
| sequence | R | |
| description | BINDING => ECO:0000269|PubMed:15215896, ECO:0007744|PDB:1RGI | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 9) | chain | A |
| residue | 117 | |
| type | BINDING | |
| sequence | V | |
| description | BINDING => ECO:0000269|PubMed:15215896, ECO:0007744|PDB:1RGI | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 10) | chain | A |
| residue | 146 | |
| type | BINDING | |
| sequence | A | |
| description | BINDING => ECO:0000269|PubMed:15215896, ECO:0007744|PDB:1RGI | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 11) | chain | A |
| residue | 303 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000269|PubMed:15215896, ECO:0007744|PDB:1RGI | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 12) | chain | A |
| residue | 304 | |
| type | BINDING | |
| sequence | C | |
| description | BINDING => ECO:0000269|PubMed:15215896, ECO:0007744|PDB:1RGI | |
| source | Swiss-Prot : SWS_FT_FI1 | |
| 13) | chain | A |
| residue | 136 | |
| type | BINDING | |
| sequence | S | |
| description | BINDING => ECO:0000250 | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 14) | chain | A |
| residue | 162 | |
| type | BINDING | |
| sequence | L | |
| description | BINDING => ECO:0000250 | |
| source | Swiss-Prot : SWS_FT_FI2 | |
| 15) | chain | A |
| residue | 495 | |
| type | BINDING | |
| sequence | V | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 16) | chain | A |
| residue | 525 | |
| type | BINDING | |
| sequence | S | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 17) | chain | A |
| residue | 565 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 18) | chain | A |
| residue | 566 | |
| type | BINDING | |
| sequence | A | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 19) | chain | A |
| residue | 588 | |
| type | BINDING | |
| sequence | K | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 20) | chain | A |
| residue | 670 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 21) | chain | A |
| residue | 671 | |
| type | BINDING | |
| sequence | V | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 22) | chain | A |
| residue | 693 | |
| type | BINDING | |
| sequence | K | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 23) | chain | A |
| residue | 210 | |
| type | BINDING | |
| sequence | R | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 24) | chain | A |
| residue | 260 | |
| type | BINDING | |
| sequence | T | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 25) | chain | A |
| residue | 445 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 26) | chain | A |
| residue | 446 | |
| type | BINDING | |
| sequence | S | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 27) | chain | A |
| residue | 476 | |
| type | BINDING | |
| sequence | V | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 28) | chain | A |
| residue | 488 | |
| type | BINDING | |
| sequence | E | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 29) | chain | A |
| residue | 493 | |
| type | BINDING | |
| sequence | T | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 30) | chain | A |
| residue | 187 | |
| type | BINDING | |
| sequence | D | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 31) | chain | A |
| residue | 188 | |
| type | BINDING | |
| sequence | C | |
| description | BINDING => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI3 | |
| 32) | chain | A |
| residue | 439 | |
| type | MOD_RES | |
| sequence | G | |
| description | Phosphotyrosine => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI4 | |
| 33) | chain | A |
| residue | 577 | |
| type | MOD_RES | |
| sequence | L | |
| description | Phosphotyrosine => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI4 | |
| 34) | chain | A |
| residue | 625 | |
| type | MOD_RES | |
| sequence | R | |
| description | Phosphotyrosine => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI4 | |
| 35) | chain | A |
| residue | 60 | |
| type | MOD_RES | |
| sequence | G | |
| description | Phosphotyrosine => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI4 | |
| 36) | chain | A |
| residue | 383 | |
| type | MOD_RES | |
| sequence | L | |
| description | Phosphotyrosine => ECO:0000250|UniProtKB:P06396 | |
| source | Swiss-Prot : SWS_FT_FI4 | |