[English] 日本語
 Yorodumi
Yorodumi- EMDB-1093: Cryo-EM visualization of a viral internal ribosome entry site bou... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1093 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
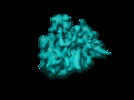
| Title | Cryo-EM visualization of a viral internal ribosome entry site bound to human ribosomes: the IRES functions as an RNA-based translation factor. | |||||||||
 Map data Map data | map | |||||||||
 Sample Sample |
| |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 18.3 Å cryo EM / Resolution: 18.3 Å | |||||||||
 Authors Authors | Spahn CM / Frank J | |||||||||
 Citation Citation |  Journal: Cell / Year: 2004 Journal: Cell / Year: 2004Title: Cryo-EM visualization of a viral internal ribosome entry site bound to human ribosomes: the IRES functions as an RNA-based translation factor. Authors: Christian M T Spahn / Eric Jan / Anke Mulder / Robert A Grassucci / Peter Sarnow / Joachim Frank /  Abstract: Internal initiation of protein synthesis in eukaryotes is accomplished by recruitment of ribosomes to structured internal ribosome entry sites (IRESs), which are located in certain viral and cellular ...Internal initiation of protein synthesis in eukaryotes is accomplished by recruitment of ribosomes to structured internal ribosome entry sites (IRESs), which are located in certain viral and cellular messenger RNAs. An IRES element in cricket paralysis virus (CrPV) can directly assemble 80S ribosomes in the absence of canonical initiation factors and initiator tRNA. Here we present cryo-EM structures of the CrPV IRES bound to the human ribosomal 40S subunit and to the 80S ribosome. The CrPV IRES adopts a defined, elongate structure within the ribosomal intersubunit space and forms specific contacts with components of the ribosomal A, P, and E sites. Conformational changes in the ribosome as well as within the IRES itself show that CrPV IRES actively manipulates the ribosome. CrPV-like IRES elements seem to act as RNA-based translation factors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1093.map.gz emd_1093.map.gz | 7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1093-v30.xml emd-1093-v30.xml emd-1093.xml emd-1093.xml | 7.5 KB 7.5 KB | Display Display |  EMDB header EMDB header |
| Images |  1093.gif 1093.gif | 43.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1093 http://ftp.pdbj.org/pub/emdb/structures/EMD-1093 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1093 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1093 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_1093.map.gz / Format: CCP4 / Size: 7.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1093.map.gz / Format: CCP4 / Size: 7.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.66 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human ribosome 80s
| Entire | Name: Human ribosome 80s |
|---|---|
| Components |
|
-Supramolecule #1000: Human ribosome 80s
| Supramolecule | Name: Human ribosome 80s / type: sample / ID: 1000 / Number unique components: 1 |
|---|
-Supramolecule #1: Human ribosome 80s
| Supramolecule | Name: Human ribosome 80s / type: complex / ID: 1 / Recombinant expression: No / Ribosome-details: ribosome-eukaryote: ALL |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) / synonym: Human Homo sapiens (human) / synonym: Human |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Vitrification | Cryogen name: ETHANE |
|---|
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F30 |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 38000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.26 mm / Nominal magnification: 39000 Bright-field microscopy / Cs: 2.26 mm / Nominal magnification: 39000 |
| Sample stage | Specimen holder: FEI Polara cartridge system / Specimen holder model: OTHER |
| Temperature | Average: 84 K |
| Alignment procedure | Legacy - Astigmatism: objective lens astigmatism was corrected at 100,000 times magnification |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: ZEISS SCAI / Digitization - Sampling interval: 14 µm / Average electron dose: 20 e/Å2 |
| Tilt angle min | 0 |
| Tilt angle max | 0 |
| Experimental equipment |  Model: Tecnai F30 / Image courtesy: FEI Company |
- Image processing
Image processing
| CTF correction | Details: defocus group volumes |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 18.3 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: SPIDER |
 Movie
Movie Controller
Controller