[English] 日本語
 Yorodumi
Yorodumi- EMDB-3086: negative stain EM of BG505 SOSIP.664 in complex with sCD4, 17b, a... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3086 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
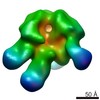
| Title | negative stain EM of BG505 SOSIP.664 in complex with sCD4, 17b, and 8ANC195 | |||||||||
 Map data Map data | negative stain EM single particle analysis. The particles were picked using EMAN2. CTF correction was done using EMAN2. 2D and 3D classification was done using Relion to sort particles. The refinement and gold-standard FSC calculation was done using Relion. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | HIV-1 Env trimer /  broadly neutralizing antibody broadly neutralizing antibody | |||||||||
| Function / homology |  Function and homology information Function and homology informationhelper T cell enhancement of adaptive immune response /  interleukin-16 binding / interleukin-16 receptor activity / maintenance of protein location in cell / interleukin-16 binding / interleukin-16 receptor activity / maintenance of protein location in cell /  T cell selection / MHC class II protein binding / cellular response to granulocyte macrophage colony-stimulating factor stimulus / interleukin-15-mediated signaling pathway / positive regulation of monocyte differentiation / Nef Mediated CD4 Down-regulation ...helper T cell enhancement of adaptive immune response / T cell selection / MHC class II protein binding / cellular response to granulocyte macrophage colony-stimulating factor stimulus / interleukin-15-mediated signaling pathway / positive regulation of monocyte differentiation / Nef Mediated CD4 Down-regulation ...helper T cell enhancement of adaptive immune response /  interleukin-16 binding / interleukin-16 receptor activity / maintenance of protein location in cell / interleukin-16 binding / interleukin-16 receptor activity / maintenance of protein location in cell /  T cell selection / MHC class II protein binding / cellular response to granulocyte macrophage colony-stimulating factor stimulus / interleukin-15-mediated signaling pathway / positive regulation of monocyte differentiation / Nef Mediated CD4 Down-regulation / Alpha-defensins / positive regulation of kinase activity / T cell selection / MHC class II protein binding / cellular response to granulocyte macrophage colony-stimulating factor stimulus / interleukin-15-mediated signaling pathway / positive regulation of monocyte differentiation / Nef Mediated CD4 Down-regulation / Alpha-defensins / positive regulation of kinase activity /  regulation of T cell activation / regulation of T cell activation /  T cell receptor complex / extracellular matrix structural constituent / Other interleukin signaling / T cell receptor complex / extracellular matrix structural constituent / Other interleukin signaling /  enzyme-linked receptor protein signaling pathway / Translocation of ZAP-70 to Immunological synapse / Phosphorylation of CD3 and TCR zeta chains / Dectin-2 family / regulation of calcium ion transport / macrophage differentiation / Generation of second messenger molecules / T cell differentiation / PD-1 signaling / positive regulation of protein kinase activity / Binding and entry of HIV virion / enzyme-linked receptor protein signaling pathway / Translocation of ZAP-70 to Immunological synapse / Phosphorylation of CD3 and TCR zeta chains / Dectin-2 family / regulation of calcium ion transport / macrophage differentiation / Generation of second messenger molecules / T cell differentiation / PD-1 signaling / positive regulation of protein kinase activity / Binding and entry of HIV virion /  coreceptor activity / positive regulation of calcium-mediated signaling / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / coreceptor activity / positive regulation of calcium-mediated signaling / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering /  protein tyrosine kinase binding / positive regulation of establishment of T cell polarity / positive regulation of interleukin-2 production / virus-mediated perturbation of host defense response / protein tyrosine kinase binding / positive regulation of establishment of T cell polarity / positive regulation of interleukin-2 production / virus-mediated perturbation of host defense response /  T cell activation / host cell endosome membrane / calcium-mediated signaling / Vpu mediated degradation of CD4 / clathrin-coated endocytic vesicle membrane / T cell activation / host cell endosome membrane / calcium-mediated signaling / Vpu mediated degradation of CD4 / clathrin-coated endocytic vesicle membrane /  cell surface receptor protein tyrosine kinase signaling pathway / positive regulation of T cell activation / positive regulation of peptidyl-tyrosine phosphorylation / transmembrane signaling receptor activity / Cargo recognition for clathrin-mediated endocytosis / Downstream TCR signaling / virus receptor activity / cell surface receptor protein tyrosine kinase signaling pathway / positive regulation of T cell activation / positive regulation of peptidyl-tyrosine phosphorylation / transmembrane signaling receptor activity / Cargo recognition for clathrin-mediated endocytosis / Downstream TCR signaling / virus receptor activity /  signaling receptor activity / signaling receptor activity /  Clathrin-mediated endocytosis / MHC class II protein complex binding / clathrin-dependent endocytosis of virus by host cell / positive regulation of canonical NF-kappaB signal transduction / defense response to Gram-negative bacterium / Clathrin-mediated endocytosis / MHC class II protein complex binding / clathrin-dependent endocytosis of virus by host cell / positive regulation of canonical NF-kappaB signal transduction / defense response to Gram-negative bacterium /  adaptive immune response / positive regulation of MAPK cascade / positive regulation of viral entry into host cell / positive regulation of ERK1 and ERK2 cascade / cell surface receptor signaling pathway / adaptive immune response / positive regulation of MAPK cascade / positive regulation of viral entry into host cell / positive regulation of ERK1 and ERK2 cascade / cell surface receptor signaling pathway /  viral protein processing / viral protein processing /  early endosome / early endosome /  cell adhesion / cell adhesion /  immune response / positive regulation of protein phosphorylation / immune response / positive regulation of protein phosphorylation /  membrane raft / fusion of virus membrane with host plasma membrane / membrane raft / fusion of virus membrane with host plasma membrane /  endoplasmic reticulum lumen / external side of plasma membrane / fusion of virus membrane with host endosome membrane / endoplasmic reticulum lumen / external side of plasma membrane / fusion of virus membrane with host endosome membrane /  viral envelope / viral envelope /  lipid binding / structural molecule activity / virion attachment to host cell / endoplasmic reticulum membrane / lipid binding / structural molecule activity / virion attachment to host cell / endoplasmic reticulum membrane /  protein kinase binding / host cell plasma membrane / virion membrane / positive regulation of DNA-templated transcription / protein kinase binding / host cell plasma membrane / virion membrane / positive regulation of DNA-templated transcription /  enzyme binding / enzyme binding /  signal transduction / protein homodimerization activity / zinc ion binding / signal transduction / protein homodimerization activity / zinc ion binding /  membrane / identical protein binding / membrane / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |    Human immunodeficiency virus 1 / Human immunodeficiency virus 1 /   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  negative staining / Resolution: 16.8 Å negative staining / Resolution: 16.8 Å | |||||||||
 Authors Authors | Scharf L / Wang H / Gao H / Chen S / McDowall A / Bjorkman P | |||||||||
 Citation Citation |  Journal: Cell / Year: 2015 Journal: Cell / Year: 2015Title: Broadly Neutralizing Antibody 8ANC195 Recognizes Closed and Open States of HIV-1 Env. Authors: Louise Scharf / Haoqing Wang / Han Gao / Songye Chen / Alasdair W McDowall / Pamela J Bjorkman /  Abstract: The HIV-1 envelope (Env) spike contains limited epitopes for broadly neutralizing antibodies (bNAbs); thus, most neutralizing antibodies are strain specific. The 8ANC195 epitope, defined by crystal ...The HIV-1 envelope (Env) spike contains limited epitopes for broadly neutralizing antibodies (bNAbs); thus, most neutralizing antibodies are strain specific. The 8ANC195 epitope, defined by crystal and electron microscopy (EM) structures of bNAb 8ANC195 complexed with monomeric gp120 and trimeric Env, respectively, spans the gp120 and gp41 Env subunits. To investigate 8ANC195's gp41 epitope at higher resolution, we solved a 3.58 Å crystal structure of 8ANC195 complexed with fully glycosylated Env trimer, revealing 8ANC195 insertion into a glycan shield gap to contact gp120 and gp41 glycans and protein residues. To determine whether 8ANC195 recognizes the CD4-bound open Env conformation that leads to co-receptor binding and fusion, one of several known conformations of virion-associated Env, we solved EM structures of an Env/CD4/CD4-induced antibody/8ANC195 complex. 8ANC195 binding partially closed the CD4-bound trimer, confirming structural plasticity of Env by revealing a previously unseen conformation. 8ANC195's ability to bind different Env conformations suggests advantages for potential therapeutic applications. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3086.map.gz emd_3086.map.gz | 6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3086-v30.xml emd-3086-v30.xml emd-3086.xml emd-3086.xml | 14.2 KB 14.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_3086_fsc.xml emd_3086_fsc.xml | 4.9 KB | Display |  FSC data file FSC data file |
| Images |  EMD-3086.jpg EMD-3086.jpg | 1.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3086 http://ftp.pdbj.org/pub/emdb/structures/EMD-3086 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3086 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3086 | HTTPS FTP |
-Related structure data
| Related structure data |  5a7xMC  3096C  5a8hC  5cjxC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_3086.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3086.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | negative stain EM single particle analysis. The particles were picked using EMAN2. CTF correction was done using EMAN2. 2D and 3D classification was done using Relion to sort particles. The refinement and gold-standard FSC calculation was done using Relion. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.5 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : soluble HIV-1 Env trimer BG505 SOSIP.664 in complex with soluble ...
| Entire | Name: soluble HIV-1 Env trimer BG505 SOSIP.664 in complex with soluble CD4s (D1-D2), broadly neutralizing antibody 17b Fabs, and broadly neutralizing antibody 8ANC195 variant G32K5 Fabs |
|---|---|
| Components |
|
-Supramolecule #1000: soluble HIV-1 Env trimer BG505 SOSIP.664 in complex with soluble ...
| Supramolecule | Name: soluble HIV-1 Env trimer BG505 SOSIP.664 in complex with soluble CD4s (D1-D2), broadly neutralizing antibody 17b Fabs, and broadly neutralizing antibody 8ANC195 variant G32K5 Fabs type: sample / ID: 1000 Oligomeric state: 3 Fabs of 17b, 3 sCD4, and 3 Fabs of 8ANC195 G52K5 bind to Env trimer BG505 SOSIP Number unique components: 4 |
|---|---|
| Molecular weight | Theoretical: 500 KDa |
-Macromolecule #1: Soluble HIV-1 Env trimer BG505 SOSIP.664
| Macromolecule | Name: Soluble HIV-1 Env trimer BG505 SOSIP.664 / type: protein_or_peptide / ID: 1 / Name.synonym: BG505 SOSIP / Number of copies: 1 / Oligomeric state: trimer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:    Human immunodeficiency virus 1 / synonym: HIV-1 Human immunodeficiency virus 1 / synonym: HIV-1 |
| Molecular weight | Theoretical: 420 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) / Recombinant cell: HEK293-6E / Recombinant plasmid: pTT5 Homo sapiens (human) / Recombinant cell: HEK293-6E / Recombinant plasmid: pTT5 |
-Macromolecule #2: Fab of broadly neutralizing antibody 17b
| Macromolecule | Name: Fab of broadly neutralizing antibody 17b / type: protein_or_peptide / ID: 2 / Name.synonym: 17b / Number of copies: 3 / Oligomeric state: monomer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) / synonym: Human Homo sapiens (human) / synonym: Human |
| Molecular weight | Theoretical: 50 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) / Recombinant cell: HEK293-6E / Recombinant plasmid: pTT5 Homo sapiens (human) / Recombinant cell: HEK293-6E / Recombinant plasmid: pTT5 |
-Macromolecule #3: soluble CD4 (D1-D2 domains)
| Macromolecule | Name: soluble CD4 (D1-D2 domains) / type: protein_or_peptide / ID: 3 / Name.synonym: sCD4 / Number of copies: 3 / Oligomeric state: monomer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) / synonym: Human Homo sapiens (human) / synonym: Human |
| Molecular weight | Theoretical: 20 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) / Recombinant cell: HEK293-6E / Recombinant plasmid: pTT5 Homo sapiens (human) / Recombinant cell: HEK293-6E / Recombinant plasmid: pTT5 |
-Macromolecule #4: Fab of broadly neutralizing antibody 8ANC195 variant G52K5
| Macromolecule | Name: Fab of broadly neutralizing antibody 8ANC195 variant G52K5 type: protein_or_peptide / ID: 4 / Name.synonym: 8ANC195 G52K5 / Number of copies: 3 / Oligomeric state: monomer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) / synonym: Human Homo sapiens (human) / synonym: Human |
| Molecular weight | Theoretical: 50 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) / Recombinant cell: HEK293-6E / Recombinant plasmid: pTT5 Homo sapiens (human) / Recombinant cell: HEK293-6E / Recombinant plasmid: pTT5 |
-Experimental details
-Structure determination
| Method |  negative staining negative staining |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.01 mg/mL |
|---|---|
| Buffer | pH: 8 / Details: 20mM Tris, 50mM NaCl |
| Staining | Type: NEGATIVE Details: Grids with adsorbed protein cross-linked by glutaraldehyde vapor and stained by 3% w/v uranyl acetate for 30 seconds. |
| Grid | Details: glow discharged ultrathin C film on holey carbon support film, 400 mesh, Cu grids |
| Vitrification | Cryogen name: NONE / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI 12 |
|---|---|
| Electron beam | Acceleration voltage: 120 kV / Electron source: LAB6 |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.2 mm / Nominal magnification: 42000 Bright-field microscopy / Cs: 2.2 mm / Nominal magnification: 42000 |
| Sample stage | Specimen holder model: SIDE ENTRY, EUCENTRIC |
| Date | Apr 7, 2015 |
| Image recording | Category: CCD / Film or detector model: GATAN ULTRASCAN 1000 (2k x 2k) / Number real images: 642 |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - #0 - Chain ID: G / Chain - #1 - Chain ID: C / Chain - #2 - Chain ID: L / Chain - #3 - Chain ID: H |
|---|---|
| Software | Name:  Chimera Chimera |
| Details | Fitted by manual docking first and then using Chimera with the following options: real-time correlation/average update, and use map simulated from atoms, resolution 15 A |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
| Output model |  PDB-5a7x: |
-Atomic model buiding 2
| Initial model | PDB ID: Chain - #0 - Chain ID: L / Chain - #1 - Chain ID: H |
|---|---|
| Software | Name:  Chimera Chimera |
| Details | Fitted by manual docking first and then using Chimera with the following options: real-time correlation/average update, and use map simulated from atoms, resolution 15 A |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
| Output model |  PDB-5a7x: |
 Movie
Movie Controller
Controller