[English] 日本語
 Yorodumi
Yorodumi- EMDB-1765: Single particle analysis of the 1:1 complex of PSD-95 and Kir2.1N... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1765 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
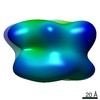
| Title | Single particle analysis of the 1:1 complex of PSD-95 and Kir2.1NC_4 in negative stain | |||||||||
 Map data Map data | Negative stain EM map of the complex of PSD-95 and Kir2.1NC | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  Scaffold protein / membrane associated / Guanylatekinase / PDZ / SH3 / Scaffold protein / membrane associated / Guanylatekinase / PDZ / SH3 /  Ion Channel / Ion Channel /  cytoplasmic domain / inwardly rectifying / cytoplasmic domain / inwardly rectifying /  membrane protein / membrane protein /  homotetramer homotetramer | |||||||||
| Function / homology |  Potassium channel, inwardly rectifying, Kir2.1 / neuronal ion channel clustering / Disks large 1-like / membrane => GO:0016020 Potassium channel, inwardly rectifying, Kir2.1 / neuronal ion channel clustering / Disks large 1-like / membrane => GO:0016020 Function and homology information Function and homology information | |||||||||
| Biological species |   Mus musculus (house mouse) / Mus musculus (house mouse) /   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  negative staining / Resolution: 20.5 Å negative staining / Resolution: 20.5 Å | |||||||||
 Authors Authors | Fomina S / Howard TD / Sleator OK / Golovanova M / O'Ryan L / Leyland M / Grossmann JG / Collins RF / Prince SM | |||||||||
 Citation Citation |  Journal: Biochim Biophys Acta / Year: 2011 Journal: Biochim Biophys Acta / Year: 2011Title: Self-directed assembly and clustering of the cytoplasmic domains of inwardly rectifying Kir2.1 potassium channels on association with PSD-95. Authors: Svetlana Fomina / Tina D Howard / Olivia K Sleator / Marina Golovanova / Liam O'Ryan / Mark L Leyland / J Günter Grossmann / Richard F Collins / Stephen M Prince /  Abstract: The interaction of the extra-membranous domain of tetrameric inwardly rectifying Kir2.1 ion channels (Kir2.1NC(4)) with the membrane associated guanylate kinase protein PSD-95 has been studied using ...The interaction of the extra-membranous domain of tetrameric inwardly rectifying Kir2.1 ion channels (Kir2.1NC(4)) with the membrane associated guanylate kinase protein PSD-95 has been studied using Transmission Electron Microscopy in negative stain. Three types of complexes were observed in electron micrographs corresponding to a 1:1 complex, a large self-enclosed tetrad complex and extended chains of linked channel domains. Using models derived from small angle X-ray scattering experiments in which high resolution structures from X-ray crystallographic and Nuclear Magnetic Resonance studies are positioned, the envelopes from single particle analysis can be resolved as a Kir2.1NC(4):PSD-95 complex and a tetrad of this unit (Kir2.1NC(4):PSD-95)(4). The tetrad complex shows the close association of the Kir2.1 cytoplasmic domains and the influence of PSD-95 mediated self-assembly on the clustering of these channels. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1765.map.gz emd_1765.map.gz | 214.7 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1765-v30.xml emd-1765-v30.xml emd-1765.xml emd-1765.xml | 12.5 KB 12.5 KB | Display Display |  EMDB header EMDB header |
| Images |  image1765.png image1765.png | 148.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1765 http://ftp.pdbj.org/pub/emdb/structures/EMD-1765 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1765 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1765 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_1765.map.gz / Format: CCP4 / Size: 422.9 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1765.map.gz / Format: CCP4 / Size: 422.9 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Negative stain EM map of the complex of PSD-95 and Kir2.1NC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.667 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Complex of mouse Kir2.1, cytoplamic domain, homotetramer of fused...
| Entire | Name: Complex of mouse Kir2.1, cytoplamic domain, homotetramer of fused N,C termini with rat PSD-95 |
|---|---|
| Components |
|
-Supramolecule #1000: Complex of mouse Kir2.1, cytoplamic domain, homotetramer of fused...
| Supramolecule | Name: Complex of mouse Kir2.1, cytoplamic domain, homotetramer of fused N,C termini with rat PSD-95 type: sample / ID: 1000 Details: Multi-refinement was undertaken to separate Kir2.1NC_4 alone from the complex Oligomeric state: One tetramer of Kir2.1NC binds to PSD-95 / Number unique components: 2 |
|---|---|
| Molecular weight | Theoretical: 218 KDa / Method: Sum of component MW |
-Macromolecule #1: Kir2.1 cytoplasmic domain
| Macromolecule | Name: Kir2.1 cytoplasmic domain / type: protein_or_peptide / ID: 1 / Name.synonym: Kir2.1NC / Number of copies: 4 / Oligomeric state: Tetramer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) / synonym: Mouse / Location in cell: Membrane Mus musculus (house mouse) / synonym: Mouse / Location in cell: Membrane |
| Molecular weight | Experimental: 140 KDa / Theoretical: 140 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) / Recombinant plasmid: pET15b Escherichia coli (E. coli) / Recombinant plasmid: pET15b |
| Sequence | GO: membrane => GO:0016020 / InterPro:  Potassium channel, inwardly rectifying, Kir2.1 Potassium channel, inwardly rectifying, Kir2.1 |
-Macromolecule #2: PSD-95
| Macromolecule | Name: PSD-95 / type: protein_or_peptide / ID: 2 / Name.synonym: PSD-95 / Number of copies: 1 / Oligomeric state: Monomer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:   Rattus norvegicus (Norway rat) / synonym: Rat Rattus norvegicus (Norway rat) / synonym: Rat |
| Molecular weight | Experimental: 95 KDa / Theoretical: 78 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) / Recombinant plasmid: pGEX-6P Escherichia coli (E. coli) / Recombinant plasmid: pGEX-6P |
| Sequence | GO: neuronal ion channel clustering / InterPro: Disks large 1-like |
-Experimental details
-Structure determination
| Method |  negative staining negative staining |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.5 Details: 20mM Tris/HCl, 150mM NaCl, 1mM reduced GSH, 1mM EDTA, 50mM L-Glutamic acid, 50mM L-Arginine |
| Staining | Type: NEGATIVE Details: Samples were adsorbed onto freshly glow discharged carbon film grids. Sample solution was pipetted into the grid followed by blotting, de-ionized water was then applied for 10s followed by ...Details: Samples were adsorbed onto freshly glow discharged carbon film grids. Sample solution was pipetted into the grid followed by blotting, de-ionized water was then applied for 10s followed by blotting, 2%w/v Uranyl Acetate solution was applied followed by a final blotting step. |
| Grid | Details: 400 mesh Copper |
| Vitrification | Cryogen name: NONE / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI 10 |
|---|---|
| Electron beam | Acceleration voltage: 100 kV / Electron source: TUNGSTEN HAIRPIN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 3.6 mm / Nominal defocus max: 1.75 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 43000 Bright-field microscopy / Cs: 3.6 mm / Nominal defocus max: 1.75 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 43000 |
| Sample stage | Specimen holder: Eucentric / Specimen holder model: PHILIPS ROTATION HOLDER |
| Details | Low dose |
| Image recording | Category: FILM / Film or detector model: GENERIC FILM / Digitization - Scanner: OTHER / Digitization - Sampling interval: 15 µm / Number real images: 15 / Od range: 2 / Bits/pixel: 8 |
| Tilt angle min | 0 |
| Tilt angle max | 0 |
- Image processing
Image processing
| CTF correction | Details: Parameters determined using a calculated Scattering curve |
|---|---|
| Final two d classification | Number classes: 84 |
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 20.5 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: EMAN / Number images used: 24199 |
| Details | Particles initially selected using automated particle picking based on a subset of representative particles on each micrograph. |
-Atomic model buiding 1
| Initial model | PDB ID: |
|---|---|
| Software | Name:  Chimera Chimera |
| Details | ROTATION MATRIX 0.62166 0.76947 -0.14647 0.00842 0.18041 0.98355 0.78325 -0.61267 0.10567 TRANSLATION VECTOR IN AS -7.89876 -3.87420 -3.22822 |
| Refinement | Space: REAL |
-Atomic model buiding 2
| Initial model | PDB ID: |
|---|---|
| Software | Name:  Chimera Chimera |
| Details | ROTATION MATRIX 0.31682 0.16747 0.93358 -0.39203 -0.87316 0.28967 0.86368 -0.45776 -0.21099 TRANSLATION VECTOR IN AS -6.39213 21.17728 3.96066 |
| Refinement | Space: REAL |
 Movie
Movie Controller
Controller