+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8wrz | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cry-EM structure of cannabinoid receptor-arrestin 2 complex | ||||||||||||||||||
 Components Components |
| ||||||||||||||||||
 Keywords Keywords |  MEMBRANE PROTEIN / MEMBRANE PROTEIN /  complex / complex /  GPCR GPCR | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationrenal water retention / Defective AVP does not bind AVPR2 and causes neurohypophyseal diabetes insipidus (NDI) / cannabinoid signaling pathway /  regulation of penile erection / retrograde trans-synaptic signaling by endocannabinoid / Vasopressin-like receptors / regulation of systemic arterial blood pressure by vasopressin / regulation of penile erection / retrograde trans-synaptic signaling by endocannabinoid / Vasopressin-like receptors / regulation of systemic arterial blood pressure by vasopressin /  vasopressin receptor activity / vasopressin receptor activity /  cannabinoid receptor activity / cannabinoid receptor activity /  angiotensin receptor binding ...renal water retention / Defective AVP does not bind AVPR2 and causes neurohypophyseal diabetes insipidus (NDI) / cannabinoid signaling pathway / angiotensin receptor binding ...renal water retention / Defective AVP does not bind AVPR2 and causes neurohypophyseal diabetes insipidus (NDI) / cannabinoid signaling pathway /  regulation of penile erection / retrograde trans-synaptic signaling by endocannabinoid / Vasopressin-like receptors / regulation of systemic arterial blood pressure by vasopressin / regulation of penile erection / retrograde trans-synaptic signaling by endocannabinoid / Vasopressin-like receptors / regulation of systemic arterial blood pressure by vasopressin /  vasopressin receptor activity / vasopressin receptor activity /  cannabinoid receptor activity / cannabinoid receptor activity /  angiotensin receptor binding / negative regulation of mast cell activation / trans-synaptic signaling by endocannabinoid, modulating synaptic transmission / negative regulation of fatty acid beta-oxidation / negative regulation of dopamine secretion / positive regulation of acute inflammatory response to antigenic stimulus / angiotensin receptor binding / negative regulation of mast cell activation / trans-synaptic signaling by endocannabinoid, modulating synaptic transmission / negative regulation of fatty acid beta-oxidation / negative regulation of dopamine secretion / positive regulation of acute inflammatory response to antigenic stimulus /  regulation of feeding behavior / regulation of presynaptic cytosolic calcium ion concentration / negative regulation of serotonin secretion / Activation of SMO / positive regulation of systemic arterial blood pressure / regulation of feeding behavior / regulation of presynaptic cytosolic calcium ion concentration / negative regulation of serotonin secretion / Activation of SMO / positive regulation of systemic arterial blood pressure /  hemostasis / telencephalon development / negative regulation of action potential / negative regulation of interleukin-8 production / Class A/1 (Rhodopsin-like receptors) / positive regulation of blood pressure / positive regulation of fever generation / hemostasis / telencephalon development / negative regulation of action potential / negative regulation of interleukin-8 production / Class A/1 (Rhodopsin-like receptors) / positive regulation of blood pressure / positive regulation of fever generation /  regulation of metabolic process / arrestin family protein binding / G protein-coupled receptor internalization / regulation of metabolic process / arrestin family protein binding / G protein-coupled receptor internalization /  enzyme inhibitor activity / axonal fasciculation / positive regulation of intracellular signal transduction / Lysosome Vesicle Biogenesis / enzyme inhibitor activity / axonal fasciculation / positive regulation of intracellular signal transduction / Lysosome Vesicle Biogenesis /  regulation of synaptic transmission, GABAergic / positive regulation of Rho protein signal transduction / Golgi Associated Vesicle Biogenesis / negative regulation of NF-kappaB transcription factor activity / regulation of synaptic transmission, GABAergic / positive regulation of Rho protein signal transduction / Golgi Associated Vesicle Biogenesis / negative regulation of NF-kappaB transcription factor activity /  stress fiber assembly / negative regulation of Notch signaling pathway / stress fiber assembly / negative regulation of Notch signaling pathway /  pseudopodium / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / negative regulation of interleukin-6 production / regulation of insulin secretion / positive regulation of receptor internalization / endocytic vesicle / GABA-ergic synapse / maternal process involved in female pregnancy / pseudopodium / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / negative regulation of interleukin-6 production / regulation of insulin secretion / positive regulation of receptor internalization / endocytic vesicle / GABA-ergic synapse / maternal process involved in female pregnancy /  clathrin-coated pit / positive regulation of vasoconstriction / negative regulation of protein ubiquitination / clathrin-coated pit / positive regulation of vasoconstriction / negative regulation of protein ubiquitination /  regulation of synaptic transmission, glutamatergic / cellular response to hormone stimulus / regulation of synaptic transmission, glutamatergic / cellular response to hormone stimulus /  insulin-like growth factor receptor binding / activation of adenylate cyclase activity / negative regulation of blood pressure / insulin-like growth factor receptor binding / activation of adenylate cyclase activity / negative regulation of blood pressure /  visual perception / Activated NOTCH1 Transmits Signal to the Nucleus / visual perception / Activated NOTCH1 Transmits Signal to the Nucleus /  GTPase activator activity / response to nutrient / response to cocaine / response to nicotine / G protein-coupled receptor binding / response to cytokine / G protein-coupled receptor activity / GTPase activator activity / response to nutrient / response to cocaine / response to nicotine / G protein-coupled receptor binding / response to cytokine / G protein-coupled receptor activity /  peptide binding / clathrin-coated endocytic vesicle membrane / Signaling by high-kinase activity BRAF mutants / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / MAP2K and MAPK activation / cytoplasmic vesicle membrane / adenylate cyclase-activating G protein-coupled receptor signaling pathway / positive regulation of neuron projection development / peptide binding / clathrin-coated endocytic vesicle membrane / Signaling by high-kinase activity BRAF mutants / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / MAP2K and MAPK activation / cytoplasmic vesicle membrane / adenylate cyclase-activating G protein-coupled receptor signaling pathway / positive regulation of neuron projection development /  memory / Vasopressin regulates renal water homeostasis via Aquaporins / Signaling by RAF1 mutants / Signaling by moderate kinase activity BRAF mutants / Paradoxical activation of RAF signaling by kinase inactive BRAF / Signaling downstream of RAS mutants / Signaling by BRAF and RAF1 fusions / memory / Vasopressin regulates renal water homeostasis via Aquaporins / Signaling by RAF1 mutants / Signaling by moderate kinase activity BRAF mutants / Paradoxical activation of RAF signaling by kinase inactive BRAF / Signaling downstream of RAS mutants / Signaling by BRAF and RAF1 fusions /  actin cytoskeleton / Thrombin signalling through proteinase activated receptors (PARs) / actin cytoskeleton / Thrombin signalling through proteinase activated receptors (PARs) /  protein transport / Cargo recognition for clathrin-mediated endocytosis / protein transport / Cargo recognition for clathrin-mediated endocytosis /  glucose homeostasis / glucose homeostasis /  presynaptic membrane / presynaptic membrane /  Clathrin-mediated endocytosis / G alpha (i) signalling events / Clathrin-mediated endocytosis / G alpha (i) signalling events /  growth cone / ubiquitin-dependent protein catabolic process / cytoplasmic vesicle / G alpha (s) signalling events / growth cone / ubiquitin-dependent protein catabolic process / cytoplasmic vesicle / G alpha (s) signalling events /  spermatogenesis / proteasome-mediated ubiquitin-dependent protein catabolic process / response to ethanol / mitochondrial outer membrane / response to lipopolysaccharide / spermatogenesis / proteasome-mediated ubiquitin-dependent protein catabolic process / response to ethanol / mitochondrial outer membrane / response to lipopolysaccharide /  electron transfer activity / electron transfer activity /  transcription coactivator activity / transcription coactivator activity /  periplasmic space periplasmic spaceSimilarity search - Function | ||||||||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human)Phage display vector pTDisp (others)   Escherichia coli (E. coli) Escherichia coli (E. coli) | ||||||||||||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.6 Å cryo EM / Resolution: 3.6 Å | ||||||||||||||||||
 Authors Authors | Wang, Y.X. / Wang, T. / Wu, L.J. / Hua, T. / Liu, Z.J. | ||||||||||||||||||
| Funding support |  China, 5items China, 5items
| ||||||||||||||||||
 Citation Citation |  Journal: Protein Cell / Year: 2024 Journal: Protein Cell / Year: 2024Title: Cryo-EM structure of cannabinoid receptor CB1-β-arrestin complex. Authors: Yuxia Wang / Lijie Wu / Tian Wang / Junlin Liu / Fei Li / Longquan Jiang / Zhongbo Fan / Yanan Yu / Na Chen / Qianqian Sun / Qiwen Tan / Tian Hua / Zhi-Jie Liu /  | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8wrz.cif.gz 8wrz.cif.gz | 175.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8wrz.ent.gz pdb8wrz.ent.gz | 131.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8wrz.json.gz 8wrz.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/wr/8wrz https://data.pdbj.org/pub/pdb/validation_reports/wr/8wrz ftp://data.pdbj.org/pub/pdb/validation_reports/wr/8wrz ftp://data.pdbj.org/pub/pdb/validation_reports/wr/8wrz | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  37795MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein |  Arrestin ArrestinMass: 44271.508 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: ARRB1 / Production host: Homo sapiens (human) / Gene: ARRB1 / Production host:   Spodoptera frugiperda (fall armyworm) / References: UniProt: P49407 Spodoptera frugiperda (fall armyworm) / References: UniProt: P49407 |
|---|---|
| #2: Antibody | Mass: 28693.922 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.) Phage display vector pTDisp (others) / Production host:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| #3: Protein | Mass: 53577.543 Da / Num. of mol.: 1 / Mutation: M29W, H124I, T210I, E273K, T283V, R340E Source method: isolated from a genetically manipulated source Details: The chimera of Cytochrome b-562, linker, and Cannabinoid receptor 1 Source: (gene. exp.)   Escherichia coli (E. coli), (gene. exp.) Escherichia coli (E. coli), (gene. exp.)   Homo sapiens (human) Homo sapiens (human)Gene: cybC, CNR1 / Production host:   Homo sapiens (human) / Strain (production host): CNR1 / References: UniProt: P0ABE7, UniProt: P21554 Homo sapiens (human) / Strain (production host): CNR1 / References: UniProt: P0ABE7, UniProt: P21554 |
| #4: Protein/peptide |  Vasopressin receptor 2 Vasopressin receptor 2Mass: 2920.651 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: AVPR2 / Production host: Homo sapiens (human) / Gene: AVPR2 / Production host:   Spodoptera frugiperda (fall armyworm) / Strain (production host): CNR / References: UniProt: P30518 Spodoptera frugiperda (fall armyworm) / Strain (production host): CNR / References: UniProt: P30518 |
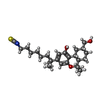
| #5: Chemical | ChemComp-8D0 / ( |
| Has ligand of interest | Y |
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Cry-EM structure of cannabinoid receptor-arrestin 2 complex Type: COMPLEX / Entity ID: #1-#4 / Source: MULTIPLE SOURCES |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Buffer solution | pH: 7.5 |
| Specimen | Conc.: 1.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm Bright-field microscopy / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm |
| Image recording | Electron dose: 60 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Num. of real images: 25847 |
- Processing
Processing
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
3D reconstruction | Resolution: 3.6 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 130058 / Symmetry type: POINT | ||||||||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj