+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8wcn | ||||||
|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of PAO1-ImcA with GMPCPP | ||||||
 Components Components | Diguanylate cyclase | ||||||
 Keywords Keywords |  MEMBRANE PROTEIN / MEMBRANE PROTEIN /  GGDEF domain / GGDEF domain /  diguanylate cyclase activity diguanylate cyclase activity | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |   Pseudomonas aeruginosa (bacteria) Pseudomonas aeruginosa (bacteria) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.2 Å cryo EM / Resolution: 3.2 Å | ||||||
 Authors Authors | Zhan, X.L. / Zhang, K. / Wang, C.C. / Fan, Q. / Tang, X.J. / Zhang, X. / Wang, K. / Fu, Y. / Liang, H.H. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: A c-di-GMP signaling module controls responses to iron in Pseudomonas aeruginosa. Authors: Xueliang Zhan / Kuo Zhang / Chenchen Wang / Qiao Fan / Xiujia Tang / Xi Zhang / Ke Wang / Yang Fu / Haihua Liang /  Abstract: Cyclic dimeric guanosine monophosphate (c-di-GMP) serves as a bacterial second messenger that modulates various processes including biofilm formation, motility, and host-microbe symbiosis. Numerous ...Cyclic dimeric guanosine monophosphate (c-di-GMP) serves as a bacterial second messenger that modulates various processes including biofilm formation, motility, and host-microbe symbiosis. Numerous studies have conducted comprehensive analysis of c-di-GMP. However, the mechanisms by which certain environmental signals such as iron control intracellular c-di-GMP levels are unclear. Here, we show that iron regulates c-di-GMP levels in Pseudomonas aeruginosa by modulating the interaction between an iron-sensing protein, IsmP, and a diguanylate cyclase, ImcA. Binding of iron to the CHASE4 domain of IsmP inhibits the IsmP-ImcA interaction, which leads to increased c-di-GMP synthesis by ImcA, thus promoting biofilm formation and reducing bacterial motility. Structural characterization of the apo-CHASE4 domain and its binding to iron allows us to pinpoint residues defining its specificity. In addition, the cryo-electron microscopy structure of ImcA in complex with a c-di-GMP analog (GMPCPP) suggests a unique conformation in which the compound binds to the catalytic pockets and to the membrane-proximal side located at the cytoplasm. Thus, our results indicate that a CHASE4 domain directly senses iron and modulates the crosstalk between c-di-GMP metabolic enzymes. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8wcn.cif.gz 8wcn.cif.gz | 163.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8wcn.ent.gz pdb8wcn.ent.gz | 126 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8wcn.json.gz 8wcn.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/wc/8wcn https://data.pdbj.org/pub/pdb/validation_reports/wc/8wcn ftp://data.pdbj.org/pub/pdb/validation_reports/wc/8wcn ftp://data.pdbj.org/pub/pdb/validation_reports/wc/8wcn | HTTPS FTP |
|---|
-Related structure data
| Related structure data | 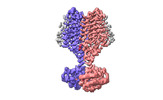 37444MC  8wctC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein |  Mass: 51329.805 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Pseudomonas aeruginosa (bacteria) / Gene: DT376_18320 / Production host: Pseudomonas aeruginosa (bacteria) / Gene: DT376_18320 / Production host:   Escherichia coli B (bacteria) / References: UniProt: A0A072ZHL9 Escherichia coli B (bacteria) / References: UniProt: A0A072ZHL9#2: Chemical | ChemComp-G2P / #3: Chemical | Has ligand of interest | N | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: GGDEF domain-containing protein / Type: COMPLEX / Entity ID: #1 / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:   Pseudomonas aeruginosa (bacteria) Pseudomonas aeruginosa (bacteria) |
| Source (recombinant) | Organism:   Escherichia coli B (bacteria) Escherichia coli B (bacteria) |
| Buffer solution | pH: 7.5 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2700 nm / Nominal defocus min: 800 nm Bright-field microscopy / Nominal defocus max: 2700 nm / Nominal defocus min: 800 nm |
| Image recording | Electron dose: 58 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
3D reconstruction | Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 205294 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj






