+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8rb4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
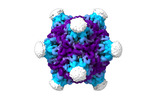
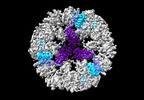
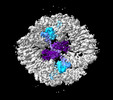
| Title | Structure of the five-fold capsomer of the PNMA2 capsid | ||||||||||||
 Components Components | Paraneoplastic antigen Ma2 homolog | ||||||||||||
 Keywords Keywords |  VIRUS LIKE PARTICLE / Endogenous retrovirus. PNMA2 / PNMA / Paraneoplastic syndrome / Paraneoplastic antigen Ma2 / VLP. VIRUS LIKE PARTICLE / Endogenous retrovirus. PNMA2 / PNMA / Paraneoplastic syndrome / Paraneoplastic antigen Ma2 / VLP. | ||||||||||||
| Function / homology | : / : / PNMA N-terminal RRM-like domain / Paraneoplastic antigen Ma / PNMA /  nucleolus / Paraneoplastic antigen Ma2 homolog nucleolus / Paraneoplastic antigen Ma2 homolog Function and homology information Function and homology information | ||||||||||||
| Biological species |   Mus musculus (house mouse) Mus musculus (house mouse) | ||||||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.2 Å cryo EM / Resolution: 3.2 Å | ||||||||||||
 Authors Authors | Erlendsson, S. / Xu, J. / Shepherd, J.D. / Briggs, J.A.G. | ||||||||||||
| Funding support |  United States, United States,  United Kingdom, United Kingdom,  Denmark, 3items Denmark, 3items
| ||||||||||||
 Citation Citation |  Journal: Cell / Year: 2024 Journal: Cell / Year: 2024Title: PNMA2 forms immunogenic non-enveloped virus-like capsids associated with paraneoplastic neurological syndrome. Authors: Junjie Xu / Simon Erlendsson / Manvendra Singh / G Aaron Holling / Matthew Regier / Iosune Ibiricu / Jenifer Einstein / Michael P Hantak / Gregory S Day / Amanda L Piquet / Tammy L Smith / ...Authors: Junjie Xu / Simon Erlendsson / Manvendra Singh / G Aaron Holling / Matthew Regier / Iosune Ibiricu / Jenifer Einstein / Michael P Hantak / Gregory S Day / Amanda L Piquet / Tammy L Smith / Stacey L Clardy / Alexandra M Whiteley / Cédric Feschotte / John A G Briggs / Jason D Shepherd /    Abstract: The paraneoplastic Ma antigen (PNMA) proteins are associated with cancer-induced paraneoplastic syndromes that present with an autoimmune response and neurological symptoms. Why PNMA proteins are ...The paraneoplastic Ma antigen (PNMA) proteins are associated with cancer-induced paraneoplastic syndromes that present with an autoimmune response and neurological symptoms. Why PNMA proteins are associated with this severe autoimmune disease is unclear. PNMA genes are predominantly expressed in the central nervous system and are ectopically expressed in some tumors. We show that PNMA2, which has been co-opted from a Ty3 retrotransposon, encodes a protein that is released from cells as non-enveloped virus-like capsids. Recombinant PNMA2 capsids injected into mice induce autoantibodies that preferentially bind external "spike" PNMA2 capsid epitopes, whereas a capsid-assembly-defective PNMA2 protein is not immunogenic. PNMA2 autoantibodies in cerebrospinal fluid of patients with anti-Ma2 paraneoplastic disease show similar preferential binding to spike capsid epitopes. PNMA2 capsid-injected mice develop learning and memory deficits. These observations suggest that PNMA2 capsids act as an extracellular antigen, capable of generating an autoimmune response that results in neurological deficits. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8rb4.cif.gz 8rb4.cif.gz | 303.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8rb4.ent.gz pdb8rb4.ent.gz | 251.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8rb4.json.gz 8rb4.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/rb/8rb4 https://data.pdbj.org/pub/pdb/validation_reports/rb/8rb4 ftp://data.pdbj.org/pub/pdb/validation_reports/rb/8rb4 ftp://data.pdbj.org/pub/pdb/validation_reports/rb/8rb4 | HTTPS FTP |
|---|
-Related structure data
| Related structure data | 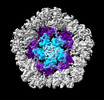 19025MC  8rb3C  8rb5C  8rb7C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 20667.789 Da / Num. of mol.: 5 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Mus musculus (house mouse) / Gene: Pnma2 / Production host: Mus musculus (house mouse) / Gene: Pnma2 / Production host:   Escherichia coli (E. coli) / References: UniProt: Q8BHK0 Escherichia coli (E. coli) / References: UniProt: Q8BHK0 |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Structure of the pentameric PNMA2 capsomer / Type: COMPLEX / Entity ID: all / Source: RECOMBINANT | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Units: MEGADALTONS / Experimental value: NO | |||||||||||||||||||||||||
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) | |||||||||||||||||||||||||
| Source (recombinant) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) | |||||||||||||||||||||||||
| Buffer solution | pH: 7.4 | |||||||||||||||||||||||||
| Buffer component |
| |||||||||||||||||||||||||
| Specimen | Conc.: 1 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES | |||||||||||||||||||||||||
| Specimen support | Film material: CARBON / Grid material: GOLD / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R2/2 | |||||||||||||||||||||||||
Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 278 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal magnification: 96000 X / Nominal defocus max: 4000 nm / Nominal defocus min: 500 nm / Cs Bright-field microscopy / Nominal magnification: 96000 X / Nominal defocus max: 4000 nm / Nominal defocus min: 500 nm / Cs : 2.7 mm / Alignment procedure: COMA FREE : 2.7 mm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 40 sec. / Electron dose: 40 e/Å2 / Detector mode: COUNTING / Film or detector model: FEI FALCON III (4k x 4k) / Num. of real images: 3005 |
| Image scans | Width: 4096 / Height: 4096 |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 2125200 | ||||||||||||||||||||||||||||||||||||||||||||||||
3D reconstruction | Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 835009 / Algorithm: FOURIER SPACE / Num. of class averages: 1 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | B value: 105 / Protocol: OTHER / Space: REAL | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Accession code: 8RB3 / Details: Full capsid fitted model / Source name: Other / Type: experimental model |
 Movie
Movie Controller
Controller



 PDBj
PDBj