[English] 日本語
 Yorodumi
Yorodumi- PDB-8phi: Crystal structure of prefusion-stabilized RSV F Variant DS-Cav1 i... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8phi | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of prefusion-stabilized RSV F Variant DS-Cav1 in complex with Lonafarnib | ||||||
 Components Components | Fusion glycoprotein F0 | ||||||
 Keywords Keywords |  VIRAL PROTEIN / RSV F Glycoprotein / VIRAL PROTEIN / RSV F Glycoprotein /  inhibitor inhibitor | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of syncytium formation by virus / host cell Golgi membrane / entry receptor-mediated virion attachment to host cell / fusion of virus membrane with host plasma membrane / host cell plasma membrane / virion membrane /  plasma membrane plasma membraneSimilarity search - Function | ||||||
| Biological species |   Human respiratory syncytial virus A2 Human respiratory syncytial virus A2 | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.289 Å MOLECULAR REPLACEMENT / Resolution: 2.289 Å | ||||||
 Authors Authors | Rajak, M. / Krey, T. | ||||||
| Funding support |  Germany, 1items Germany, 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Drug repurposing screen identifies lonafarnib as respiratory syncytial virus fusion protein inhibitor. Authors: Sake, S.M. / Zhang, X. / Rajak, M.K. / Urbanek-Quaing, M. / Carpentier, A. / Gunesch, A.P. / Grethe, C. / Matthaei, A. / Ruckert, J. / Galloux, M. / Larcher, T. / Le Goffic, R. / Hontonnou, ...Authors: Sake, S.M. / Zhang, X. / Rajak, M.K. / Urbanek-Quaing, M. / Carpentier, A. / Gunesch, A.P. / Grethe, C. / Matthaei, A. / Ruckert, J. / Galloux, M. / Larcher, T. / Le Goffic, R. / Hontonnou, F. / Chatterjee, A.K. / Johnson, K. / Morwood, K. / Rox, K. / Elgaher, W.A.M. / Huang, J. / Wetzke, M. / Hansen, G. / Fischer, N. / Eleouet, J.F. / Rameix-Welti, M.A. / Hirsch, A.K.H. / Herold, E. / Empting, M. / Lauber, C. / Schulz, T.F. / Krey, T. / Haid, S. / Pietschmann, T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8phi.cif.gz 8phi.cif.gz | 113 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8phi.ent.gz pdb8phi.ent.gz | 81 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8phi.json.gz 8phi.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ph/8phi https://data.pdbj.org/pub/pdb/validation_reports/ph/8phi ftp://data.pdbj.org/pub/pdb/validation_reports/ph/8phi ftp://data.pdbj.org/pub/pdb/validation_reports/ph/8phi | HTTPS FTP |
|---|
-Related structure data
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules F
| #1: Protein | Mass: 61581.145 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Human respiratory syncytial virus A2 / Strain: strain A2 / Production host: Human respiratory syncytial virus A2 / Strain: strain A2 / Production host:   Drosophila melanogaster (fruit fly) / References: UniProt: W8RJF9 Drosophila melanogaster (fruit fly) / References: UniProt: W8RJF9 |
|---|
-Non-polymers , 5 types, 182 molecules 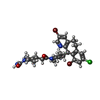







| #2: Chemical | ChemComp-336 /  Lonafarnib Lonafarnib | ||||
|---|---|---|---|---|---|
| #3: Chemical | ChemComp-SO4 /  Sulfate Sulfate | ||||
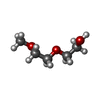
| #4: Chemical |  2-(2-Methoxyethoxy)ethanol 2-(2-Methoxyethoxy)ethanol#5: Chemical | ChemComp-CA / | #6: Water | ChemComp-HOH / |  Water Water |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.94 Å3/Da / Density % sol: 58.12 % |
|---|---|
Crystal grow | Temperature: 291.15 K / Method: vapor diffusion, sitting drop / pH: 8 / Details: PEG 6000-20%, CaCl2-400mM, & TrisHCl pH-8 100mM |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SOLEIL SOLEIL  / Beamline: PROXIMA 1 / Wavelength: 0.91983 Å / Beamline: PROXIMA 1 / Wavelength: 0.91983 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Apr 28, 2023 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.91983 Å / Relative weight: 1 : 0.91983 Å / Relative weight: 1 |
| Reflection | Resolution: 2.289→50 Å / Num. obs: 51465 / % possible obs: 99.9 % / Redundancy: 26.1 % / CC1/2: 0.997 / Rrim(I) all: 0.243 / Net I/σ(I): 10.54 |
| Reflection shell | Resolution: 2.289→2.35 Å / Mean I/σ(I) obs: 1.66 / Num. unique obs: 3770 / CC1/2: 0.882 / Rrim(I) all: 1.755 / % possible all: 99 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT / Resolution: 2.289→26.21 Å / Cor.coef. Fo:Fc: 0.899 / Cor.coef. Fo:Fc free: 0.891 / SU R Cruickshank DPI: 0.264 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.257 / SU Rfree Blow DPI: 0.194 / SU Rfree Cruickshank DPI: 0.198 MOLECULAR REPLACEMENT / Resolution: 2.289→26.21 Å / Cor.coef. Fo:Fc: 0.899 / Cor.coef. Fo:Fc free: 0.891 / SU R Cruickshank DPI: 0.264 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.257 / SU Rfree Blow DPI: 0.194 / SU Rfree Cruickshank DPI: 0.198
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 65.03 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.29 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.289→26.21 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.29→2.31 Å / Total num. of bins used: 51
|
 Movie
Movie Controller
Controller


 PDBj
PDBj





