+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8iy6 | ||||||
|---|---|---|---|---|---|---|---|
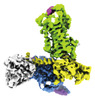
| Title | ETB-Gi complex bound to Endotheline-1, focused on receptor | ||||||
 Components Components |
| ||||||
 Keywords Keywords | PEPTIDE BINDING PROTEIN / Class A GPCR /  Endothelin / Gi / Vasoactive peptide Endothelin / Gi / Vasoactive peptide | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of prostaglandin-endoperoxide synthase activity /  endothelin A receptor binding / protein kinase C deactivation / cellular response to human chorionic gonadotropin stimulus / meiotic cell cycle process involved in oocyte maturation / phospholipase D-activating G protein-coupled receptor signaling pathway / endothelin A receptor binding / protein kinase C deactivation / cellular response to human chorionic gonadotropin stimulus / meiotic cell cycle process involved in oocyte maturation / phospholipase D-activating G protein-coupled receptor signaling pathway /  endothelin B receptor binding / rhythmic excitation / peptide hormone secretion / neural crest cell fate commitment ...positive regulation of prostaglandin-endoperoxide synthase activity / endothelin B receptor binding / rhythmic excitation / peptide hormone secretion / neural crest cell fate commitment ...positive regulation of prostaglandin-endoperoxide synthase activity /  endothelin A receptor binding / protein kinase C deactivation / cellular response to human chorionic gonadotropin stimulus / meiotic cell cycle process involved in oocyte maturation / phospholipase D-activating G protein-coupled receptor signaling pathway / endothelin A receptor binding / protein kinase C deactivation / cellular response to human chorionic gonadotropin stimulus / meiotic cell cycle process involved in oocyte maturation / phospholipase D-activating G protein-coupled receptor signaling pathway /  endothelin B receptor binding / rhythmic excitation / peptide hormone secretion / neural crest cell fate commitment / sympathetic neuron axon guidance / body fluid secretion / glomerular endothelium development / vein smooth muscle contraction / noradrenergic neuron differentiation / response to prostaglandin F / positive regulation of renal sodium excretion / leukocyte activation / positive regulation of sarcomere organization / histamine secretion / rough endoplasmic reticulum lumen / positive regulation of chemokine-mediated signaling pathway / positive regulation of odontogenesis / maternal process involved in parturition / regulation of glucose transmembrane transport / pharyngeal arch artery morphogenesis / endothelin receptor signaling pathway involved in heart process / semaphorin-plexin signaling pathway involved in axon guidance / epithelial fluid transport / podocyte differentiation / cardiac neural crest cell migration involved in outflow tract morphogenesis / negative regulation of hormone secretion / response to leptin / endothelin receptor signaling pathway / response to ozone / endothelin B receptor binding / rhythmic excitation / peptide hormone secretion / neural crest cell fate commitment / sympathetic neuron axon guidance / body fluid secretion / glomerular endothelium development / vein smooth muscle contraction / noradrenergic neuron differentiation / response to prostaglandin F / positive regulation of renal sodium excretion / leukocyte activation / positive regulation of sarcomere organization / histamine secretion / rough endoplasmic reticulum lumen / positive regulation of chemokine-mediated signaling pathway / positive regulation of odontogenesis / maternal process involved in parturition / regulation of glucose transmembrane transport / pharyngeal arch artery morphogenesis / endothelin receptor signaling pathway involved in heart process / semaphorin-plexin signaling pathway involved in axon guidance / epithelial fluid transport / podocyte differentiation / cardiac neural crest cell migration involved in outflow tract morphogenesis / negative regulation of hormone secretion / response to leptin / endothelin receptor signaling pathway / response to ozone /  Weibel-Palade body / renal sodium ion absorption / positive regulation of cell growth involved in cardiac muscle cell development / artery smooth muscle contraction / Weibel-Palade body / renal sodium ion absorption / positive regulation of cell growth involved in cardiac muscle cell development / artery smooth muscle contraction /  glomerular filtration / axonogenesis involved in innervation / positive regulation of cation channel activity / positive regulation of prostaglandin secretion / cellular response to follicle-stimulating hormone stimulus / cellular response to luteinizing hormone stimulus / regulation of pH / negative regulation of nitric-oxide synthase biosynthetic process / cellular response to mineralocorticoid stimulus / positive regulation of urine volume / negative regulation of blood coagulation / respiratory gaseous exchange by respiratory system / basal part of cell / positive regulation of smooth muscle contraction / positive regulation of hormone secretion / regulation of systemic arterial blood pressure by endothelin / glomerular filtration / axonogenesis involved in innervation / positive regulation of cation channel activity / positive regulation of prostaglandin secretion / cellular response to follicle-stimulating hormone stimulus / cellular response to luteinizing hormone stimulus / regulation of pH / negative regulation of nitric-oxide synthase biosynthetic process / cellular response to mineralocorticoid stimulus / positive regulation of urine volume / negative regulation of blood coagulation / respiratory gaseous exchange by respiratory system / basal part of cell / positive regulation of smooth muscle contraction / positive regulation of hormone secretion / regulation of systemic arterial blood pressure by endothelin /  vasoconstriction / protein kinase C-activating G protein-coupled receptor signaling pathway / embryonic heart tube development / superoxide anion generation / axon extension / dorsal/ventral pattern formation / negative regulation of protein metabolic process / positive regulation of neutrophil chemotaxis / middle ear morphogenesis / positive regulation of signaling receptor activity / cellular response to glucocorticoid stimulus / cartilage development / prostaglandin biosynthetic process / nitric oxide transport / cellular response to fatty acid / positive regulation of heart rate / cellular response to organic substance / branching involved in blood vessel morphogenesis / response to testosterone / response to dexamethasone / positive regulation of cardiac muscle hypertrophy / negative regulation of smooth muscle cell apoptotic process / vasoconstriction / protein kinase C-activating G protein-coupled receptor signaling pathway / embryonic heart tube development / superoxide anion generation / axon extension / dorsal/ventral pattern formation / negative regulation of protein metabolic process / positive regulation of neutrophil chemotaxis / middle ear morphogenesis / positive regulation of signaling receptor activity / cellular response to glucocorticoid stimulus / cartilage development / prostaglandin biosynthetic process / nitric oxide transport / cellular response to fatty acid / positive regulation of heart rate / cellular response to organic substance / branching involved in blood vessel morphogenesis / response to testosterone / response to dexamethasone / positive regulation of cardiac muscle hypertrophy / negative regulation of smooth muscle cell apoptotic process /  membrane depolarization / thyroid gland development / membrane depolarization / thyroid gland development /  regulation of vasoconstriction / positive regulation of cell size / canonical Wnt signaling pathway / response to amino acid / cellular response to interleukin-1 / positive regulation of calcium-mediated signaling / protein kinase A signaling / positive regulation of JUN kinase activity / regulation of vasoconstriction / positive regulation of cell size / canonical Wnt signaling pathway / response to amino acid / cellular response to interleukin-1 / positive regulation of calcium-mediated signaling / protein kinase A signaling / positive regulation of JUN kinase activity /  transport vesicle / ERK1 and ERK2 cascade / positive regulation of vascular associated smooth muscle cell proliferation / response to amphetamine / cellular response to transforming growth factor beta stimulus / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / response to muscle stretch / cellular response to calcium ion / mitochondrion organization / Peptide ligand-binding receptors / positive regulation of mitotic nuclear division / positive regulation of endothelial cell migration / response to activity transport vesicle / ERK1 and ERK2 cascade / positive regulation of vascular associated smooth muscle cell proliferation / response to amphetamine / cellular response to transforming growth factor beta stimulus / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / response to muscle stretch / cellular response to calcium ion / mitochondrion organization / Peptide ligand-binding receptors / positive regulation of mitotic nuclear division / positive regulation of endothelial cell migration / response to activitySimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.13 Å cryo EM / Resolution: 3.13 Å | ||||||
 Authors Authors | Sano, F.K. / Akasaka, H. / Shihoya, W. / Nureki, O. | ||||||
| Funding support |  Japan, 1items Japan, 1items
| ||||||
 Citation Citation |  Journal: Elife / Year: 2023 Journal: Elife / Year: 2023Title: Cryo-EM structure of the endothelin-1-ET-G complex. Authors: Fumiya K Sano / Hiroaki Akasaka / Wataru Shihoya / Osamu Nureki /  Abstract: The endothelin ET receptor is a promiscuous G-protein coupled receptor that is activated by vasoactive peptide endothelins. ET signaling induces reactive astrocytes in the brain and vasorelaxation in ...The endothelin ET receptor is a promiscuous G-protein coupled receptor that is activated by vasoactive peptide endothelins. ET signaling induces reactive astrocytes in the brain and vasorelaxation in vascular smooth muscle. Consequently, ET agonists are expected to be drugs for neuroprotection and improved anti-tumor drug delivery. Here, we report the cryo-electron microscopy structure of the endothelin-1-ET-G complex at 2.8 Å resolution, with complex assembly stabilized by a newly established method. Comparisons with the inactive ET receptor structures revealed how endothelin-1 activates the ET receptor. The NPxxY motif, essential for G-protein activation, is not conserved in ET, resulting in a unique structural change upon G-protein activation. Compared with other GPCR-G-protein complexes, ET binds G in the shallowest position, further expanding the diversity of G-protein binding modes. This structural information will facilitate the elucidation of G-protein activation and the rational design of ET agonists. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8iy6.cif.gz 8iy6.cif.gz | 93.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8iy6.ent.gz pdb8iy6.ent.gz | 61.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8iy6.json.gz 8iy6.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/iy/8iy6 https://data.pdbj.org/pub/pdb/validation_reports/iy/8iy6 ftp://data.pdbj.org/pub/pdb/validation_reports/iy/8iy6 ftp://data.pdbj.org/pub/pdb/validation_reports/iy/8iy6 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  35815MC  8iy5C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 67492.219 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:   Spodoptera frugiperda (fall armyworm) / Strain (production host): Sf9 cells Spodoptera frugiperda (fall armyworm) / Strain (production host): Sf9 cells |
|---|---|
| #2: Protein/peptide |  Endothelin 1 / Preproendothelin-1 / PPET1 Endothelin 1 / Preproendothelin-1 / PPET1Mass: 2497.951 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.)   Homo sapiens (human) / References: UniProt: P05305 Homo sapiens (human) / References: UniProt: P05305 |
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Complex of Endothelin-1, ETB, and Gi / Type: COMPLEX / Entity ID: all / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Buffer solution | pH: 8 |
| Specimen | Conc.: 8 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
| Specimen support | Grid material: GOLD / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R1.2/1.3 |
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal magnification: 105000 X / Nominal defocus max: 1600 nm / Nominal defocus min: 800 nm / Cs Bright-field microscopy / Nominal magnification: 105000 X / Nominal defocus max: 1600 nm / Nominal defocus min: 800 nm / Cs : 2.7 mm : 2.7 mm |
| Specimen holder | Cryogen: NITROGEN |
| Image recording | Average exposure time: 2.3 sec. / Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) / Num. of real images: 10408 |
- Processing
Processing
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
3D reconstruction | Resolution: 3.13 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 260085 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refinement | Resolution: 3.13→3.13 Å / Cor.coef. Fo:Fc: 0.877 / SU B: 20.883 / SU ML: 0.372 / ESU R: 0.39 Stereochemistry target values: MAXIMUM LIKELIHOOD WITH PHASES Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||
| Solvent computation | Solvent model: PARAMETERS FOR MASK CACLULATION | ||||||||||||||||||||||||
| Displacement parameters | Biso mean: 84.927 Å2 | ||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Total: 2495 | ||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||
| LS refinement shell | Resolution: 3→3.078 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller



 PDBj
PDBj




